Apr 24 2020
Antioxidants play a major role by protecting the living systems from the negative impacts of free radicals and, hence, they are regarded as one of the most fascinating and extensively researched compounds in the field of life sciences.
 Graphical abstract. Image Credit: Kazan Federal University.
Graphical abstract. Image Credit: Kazan Federal University.
A wide range of previously identified antioxidants and the discoveries of novel antioxidants provide opportunities to gain a deeper understanding of their mechanisms and pathways of action.
Moreover, the assessment of antioxidant ratios in different types of samples (such as herbs, foods, biological fluids, pharmaceuticals, etc.) supports quality control and helps to predict possible adverse health effects.
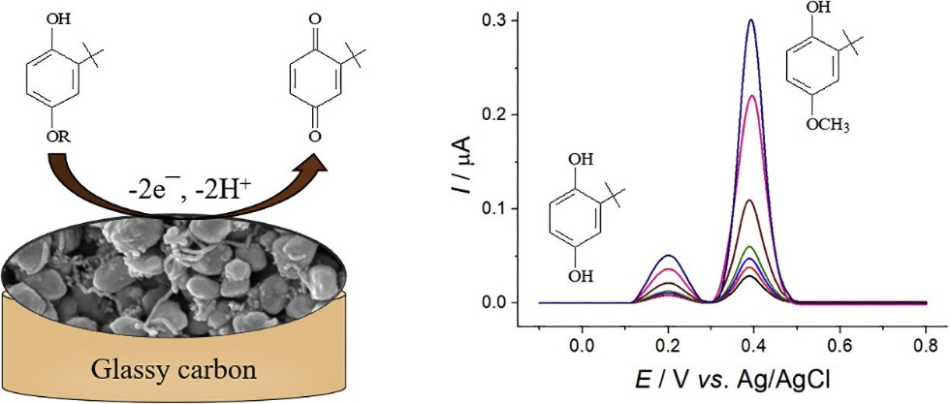
For almost two decades, Guzel Ziyatdinova and her collaborators at Kazan Federal University have been investigating the same topic. Among a range of antioxidants, synthetic phenolic antioxidants and, specifically, sterically hindered phenols have attracted plenty of interest for their practical applications. These products are utilized as food antioxidant additives for fatty and oily products to avoid oxidative rancidity. Tert-butylhydroquinone (TBHQ), butylated hydroxytoluene, and butylated hydroxyanisole (BHA) are the most frequently used preserving additives of this type of phenols.
But high concentrations of these products can cause negative health effects like the underdevelopment of the reproductive system, carcinogenesis, vision impairment, and chronic neurotoxicity, and hence their extent in food is to be meticulously controlled.
Additive mixtures can also be used and their ratios differ in different products. Hence, there is a need for selective and sensitive techniques to concurrently determine these antioxidants.
Within the configuration of compounds being analyzed, the presence of phenolic ring renders these compounds oxidizable under electrochemical oxidation as well as other kinds of conditions. Hence, electroanalysis methods can be used to determine the sterically hindered phenols.
Compared to other analytical tools, electroanalysis offers several major benefits like rapid response, high selectivity and sensitivity, cost-efficiency, simplicity, and the capacity for miniaturization.
Both BHA and TBHQ share a similar configuration and hence they are often oxidized at close potentials, making it unfeasible to determine them simultaneously. For this purpose, an innovative amperometric sensor has been developed in this study.
The sensor is based on a glassy carbon electrode enclosed with electropolymerized carminic acid as the sensitive layer and multi-walled carbon nanotubes. For the first time, the natural dye—carminic acid—was used as a monomer. Poly(carminic acid) was acquired through electrochemical potential cycling.
The conditions of electro-polymerization (the concentration of the monomer, parameters of the electrolysis, the number of cycles, and the supporting electrolyte pH) were closely monitored. These conditions significantly impact the polymeric coverage properties and, thus, the voltammetric response of the compounds of interest.
BHA and TBHQ are both oxidized on the surface of the sensor at comparatively low anodic potentials, which minimizes the interference effect from other types of electroactive compounds.
The integration of electrode surface modifiers (that is, polymeric layer and carbon nanotubes) improves the voltammetric reaction of both BHA and TBHQ and offers excellent resolution of their oxidation peaks in concurrent presence, as far as phenol oxidation continues independently. This is in complete contrast to the powerful overlapping of the oxidation peaks that occur at the unmodified glassy carbon electrode.
In the presence of α-tocopherol, ascorbic acid, and inorganic ions, the amperometric sensor is selective to BHA and TBHQ. The properties of the sensor are considerably enhanced or similar to those reported previously for another modified electrodes.
When compared to other kinds of amperometric sensors, the new sensor offers benefits like greater selectivity of BHA and TBHQ quantification and also rapidity and simplicity of its preparation.
The novel sensor has been effectively tested on linseed oil. Both BHA and TBHQ have been obtained using ultrasonic-assisted extraction with ethanol. Furthermore, the recovery values demonstrated the lack of the matrix effects, the excellent precision of the BHA and TBHQ determination, and the applicability of the sensor to actual samples. Therefore, sensors based on poly(carminic acid) can be used for maintaining the safety and quality standards of the edible oils.
The analysis demonstrated that electrodes altered with electropolymerized dyes can be used for identifying antioxidants and other structurally analogous compounds. Additional findings can be given to the enlargement of the dyes that form non-conductive polymers along with conductive nanomaterials as electrode surface modifiers.
Conversely, the analysis of other kinds of structurally analogous antioxidants employed in the food sector, or being a part of the day-to-day human diet, also holds a practical interest.
This electroanalysis challenge can be overcome by applying the above-mentioned techniques. On the whole, investigations like these will expand the application area of electrochemical techniques in the regular practice of sample screening as an excellent option to other analytical approaches, such as chromatography.
Source: https://eng.kpfu.ru/