A recent paper published in Nature Communications illustrates the importance of understanding the sensing process of metal oxide semiconductors when developing high-performance sensors. The study demonstrates that high response values of sensing materials are primarily attributed to the facile participation of lattice oxygen through density functional theory (DFT) calculations and experimental evidence, providing insights into novel sensing mechanisms.
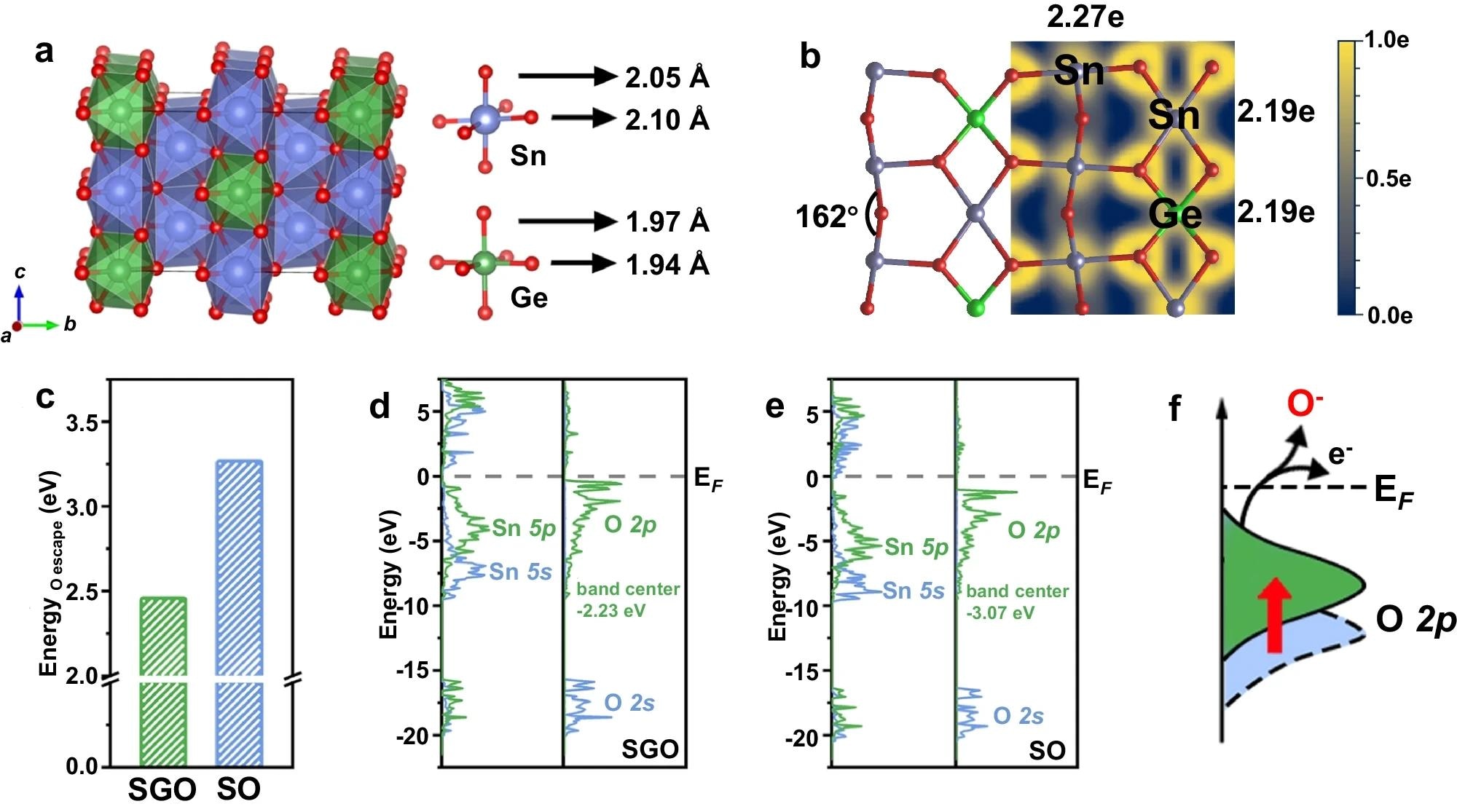 DFT Calculation. a Schematic illustration of SGO. The red, blue, and green atoms represent O, Sn, and Ge, respectively. b Electron local function (ELF) and the bader charge of SGO. c Escape energy of O atoms in SGO and SO. The electronic density of states (DOS) of SGO d and SO e, in which the Fermi level is set to 0. f The corresponding schematic illustration of the relationship between the position of O p-band (relative to Fermi level) and the conversion from surface lattice oxygen (O2-) to chemisorbed oxygen (O−). In all figures, SGO denotes Sn0.8Ge0.2O2 and SO denotes SnO2. Blue/green color corresponds to Sn/Ge throughout the Figure c and f. Image Credit: https://www.nature.com/articles/s41467-024-47078-x
DFT Calculation. a Schematic illustration of SGO. The red, blue, and green atoms represent O, Sn, and Ge, respectively. b Electron local function (ELF) and the bader charge of SGO. c Escape energy of O atoms in SGO and SO. The electronic density of states (DOS) of SGO d and SO e, in which the Fermi level is set to 0. f The corresponding schematic illustration of the relationship between the position of O p-band (relative to Fermi level) and the conversion from surface lattice oxygen (O2-) to chemisorbed oxygen (O−). In all figures, SGO denotes Sn0.8Ge0.2O2 and SO denotes SnO2. Blue/green color corresponds to Sn/Ge throughout the Figure c and f. Image Credit: https://www.nature.com/articles/s41467-024-47078-x
Efficient Hydrogen Sensors for the Safe Operation of Vehicles
Hydrogen (H2), a cost-effective energy carrier derived from renewable resources, is gaining traction in various applications, including new energy vehicles. However, the high volatility and explosive nature of hydrogen pose significant challenges for the commercialization of hydrogen vehicles, highlighting the need for advanced on-board safety sensors. Therefore, the development of high-performance hydrogen sensors is crucial for the safe operation of hydrogen vehicles.
Metal oxide semiconductor gas sensors are highly promising due to their affordability, compact size, and stability compared to other types of sensors. However, the complexity of the gas sensing technique means that their sensing mechanism is not yet fully understood, which hinders the practical development of these semiconductor materials.
The commonly accepted model for hydrogen sensing suggests that chemisorbed oxygen on the sensor's surface interacts with hydrogen in a redox reaction. However, recent evidence indicates that the effectiveness of gas sensing remains consistent, even without surface-adsorbed oxygen. Additionally, in some cases, high levels of surface-adsorbed oxygen do not enhance sensing efficiency, pointing to a more complex underlying mechanism.
Fabrication of Nanofibres
In this study, germanium-doped tin dioxide (SGO) was synthesized using the electrospinning technique to explore the role of lattice oxygen in hydrogen sensing. The precursors used in the synthesis were tin(IV) tetrachloride pentahydrate (SnCl4·5H2O) and bis(2-carboxyethyl germanium(IV) sesquioxide) (Ge-132).
Initially, SnCl4·5H2O, N,N-Dimethylformamide, and ethanol were systematically mixed. Next, bis(2-carboxyethyl germanium(IV) sesquioxide) was added and stirred into the solution until dissolved. Finally, the mixture was transferred to a medical syringe, and electrospinning was performed under a voltage of 22 kV, with specific temperature and humidity conditions maintained.
The prepared samples were analyzed using various techniques, including scanning electron microscopy (SEM), X-Ray diffraction (XRD), X-Ray photoelectron spectroscopy (XPS), and transmission electron microscopy (TEM). Additionally, density functional theory (DFT) calculations were carried out using the Vienna ab initio simulation package (VASP5.4.4).
Results and Observations
The XRD patterns revealed significant minor lattice shrinkage and peak broadening in SGO, likely due to the smaller grain size of tin dioxide (SO) caused by the incorporation of germanium species. This suggests an electronic interaction between SO and germanium. Similarly, DFT studies indicated that the addition of germanium causes distortion in the SGO lattice, increasing the likelihood of defect formation.
According to XPS results, the valence of tin species in SGO increased. Therefore, it is evident that higher valence tin species can catalyze the oxidation of H2 during the gas sensing process, which improves the response speed of SGO. To further assess these findings, a compact semiconductor gas sensor comprising SO and SGO was developed to test its hydrogen sensing performance.
The SGO sensor exhibited its best performance at 500 ppm of H2 and a temperature of 220 °C, where the response initially increased with rising temperatures before declining. Furthermore, the response of the SGO was assessed across various hydrogen concentrations at the same temperature, showing a linear relationship between concentration and response. Notably, the sensor achieved a fast recovery time of less than 58 seconds and a response time of less than 2 seconds at 1000 ppm H2.
The sensor's selectivity was assessed against six interfering gases to determine its practical applicability. It displayed a strong affinity for H2, registering a response value seven times higher than for any other gas. This exceptional selectivity is attributed to the improved reducibility of H2 and the enhanced adsorption capacity of the SGO surface.
The effects of adding Ge atoms on the oxygen escape energy in SO and the causes of this shift were investigated with the help of DFT calculations. Lattice oxygen generates chemisorbed oxygen more easily in SGO due to the significantly lower oxygen escape energy than SO.
Electronic density of states calculations reveals that the O 2p-band center in SGO is higher than in SO, suggesting a more effective transition from lattice oxygen to chemisorbed oxygen in SGO. These results align well with experimental evidence, demonstrating an easier release of surface lattice oxygen in SGO, which enhances its activity in gas sensing.
Conclusions
In summary, this research advances our understanding of gas-sensing mechanisms and underscores the importance of hydrogen-sensing materials. The team effectively demonstrated in situ characterizations that reveal the transformation of lattice oxygen into chemisorbed oxygen, actively participating in the sensing process.
In addition, DFT calculations indicate that the position of the O p-band center significantly influences the reactivity of lattice oxygen. Specifically, an upward shift promotes the escape of surface lattice oxygen, thereby enhancing H2 sensing in germanium-doped SO. Notably, the SGO-based sensor demonstrated exceptional stability, maintaining consistent response and resistance across ten cycles—an essential feature for practical applications.
Journal Reference
Li, J., Si, W., Shi, L., et al. Essential role of lattice oxygen in hydrogen sensing reaction. Nat Commun 15, 2998 (2024). https://doi.org/10.1038/s41467-024-47078-x, https://www.nature.com/articles/s41467-024-47078-x