Mar 17 2010
BioVigil LLC has recently launched the second-generation BioVigil hand hygiene monitoring system.
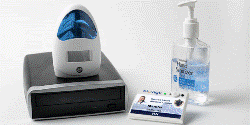 BioVigil Alcohol Sensing Technology
BioVigil Alcohol Sensing Technology
The monitoring system will help hospitals to counter effectively hospital-acquired infections (HAIs) by ensuring that healthcare personnels are complying to hand hygiene practices.
Over $20-30 billion per year is wasted on HAIs. In addition, about 1 out of 10 patients in hospitals pick up secondary infection during their stay in hospitals. Such infections are harmful for patients who are recovering from surgery, with about 99,000 a deaths a year in the U.S. caused by HAIs.
The U.S. Centers for Disease Control (CDC) has informed that hand hygiene is the most effective means for combating infections acquired in hospitals. This fact has made 34 states to incorporate standards that need hospitals to enhance their hand hygiene measures.
The recently launched BioVigil system includes sophisticated badge technology and associated sensors for effectively monitoring compliance of hand hygiene in hospitals. The system authenticates the utilization of hand sanitizer in each patient’s room, and also records compliance data into a secure database.
Brian Sheahan, CEO for BioVigil LLC, revealed that the new system has overcome the accuracy problems noticed in RF-based sensors. Sheahan explained that the RF-based system lacks the sensitivity and precision provided by the second-generation BioVigil hand monitoring system. Shehan claimed that BioVigil knows with 100 percent precision whenever any healthcare personnel entered and left a location.
Sheahan added that BioVigil is also using an alcohol sensor in the healthcare worker’s ID badge. This will make sure a doctor or nurse to enter the patient’s room and utilizes the hand sanitizer before approaching and interacting with the patient. Shehan claimed that BioVigil has designed a sensor network that renders the system patient- and user-friendly. Shehan informed that the ID badge is embedded with bright red and green LEDs for enabling patients and other workers to observe whether a healthcare worker is complying with hand hygiene technique.
Shehan revealed that BioVigil had finished an IRB-approved trial along with VCU Medical Center in September 2009, thereby authenticating the successful functioning of its second-generation BV-140 technology. Dr. Michael Edmond from the VCU Medical Center will present the trial results on March 21, 2010 at the Society for Healthcare Epidemiology of America (SHEA) conference in Atlanta, GA.
Source: http://www.biovigilsystems.com/