Having good nitrogen safety awareness is crucial when handling liquid nitrogen (LN2). Due to its extremely low temperature, LN2 is considered a dangerous substance. This has led the Occupational Safety and Health Administration (OSHA) to publish a list of nitrogen safety requirements when working with nitrogen in its liquid state.

Image Credit: CO2Meter
What is Liquid Nitrogen?
Nitrogen is a noble gas classified as inert because it does not form chemical compounds with other molecules. Nitrogen is safe for use in the food industry, as it is odorless, colorless, and tasteless. Moreover, in its liquid state, nitrogen is relatively easy to transport in tanks or cylinders.
Liquid nitrogen’s most unique and practical property is that it is extremely cold. With a boiling point of -320 °F (-196 °C), any temperature above transforms it into a gas, making it the ideal coolant. This is achieved by piping LN (liquid nitrogen) around or into other gases or objects to cool them. LN2 is also used for freezing materials in other applications.
Can You Use Liquid Nitrogen to Freeze Dry Food?
The food industry often leverages the extremely cold properties of liquid nitrogen, especially in food-freezing applications. Food-grade liquid nitrogen has several advantages over conventional mechanical freezing- or refrigeration-based processes.
In fact, liquid nitrogen is not only faster but also more flexible and has a smaller spatial footprint. Where food quality is concerned, liquid nitrogen prevents hydration loss, allowing food products to conserve their moisture and, as a result, create greater flavors.
In addition, liquid nitrogen can be used to preserve food and protect the nutrients because oxygen can oxidize the food material and ingredients. In certain circumstances, liquid nitrogen is used to enhance the atmosphere of packaging to ensure the products remain safe and high quality for the end consumer.
Benefits of Liquid Nitrogen for Food Freezing?
With liquid nitrogen being commonplace in the food industry, it is quickly becoming the ‘go-to’ coolant as it offers many benefits when it comes to freezing and powdering products. While unimaginable until fairly recently, a few benefits that liquid nitrogen provides include:
- Rapid Freezing: The extremely low temperatures of liquid nitrogen, around -320 °F (-196 °C), facilitate rapid freezing of food products. This minimizes the formation of ice crystals and preserves the overall quality of the food.
- Preservation of Texture and Nutrients: The rapid liquid nitrogen freezing process helps preserve the texture, color, and nutritional content of the food. This is vital when freezing delicate food items like fruits, vegetables, and seafood.
- Extended Shelf Life: Liquid nitrogen freezing helps extend the shelf life of food products by preventing the growth of microorganisms and enzymes. This is important when preserving perishable items and also helps limit food waste.
- Improved Product Quality: The rapid freezing process results in smaller ice crystal formation, which limits cell damage in the food. This makes for better texture and taste after the food has been thawed and prepared.
- Flexible Packaging Options: Liquid nitrogen freezing opens the door to a wider range of packaging options, including individually quick frozen (IQF) items. This allows manufacturers to package and store food in convenient portions, which gives producers and consumers greater flexibility.
- Energy Efficiency: Liquid nitrogen freezing systems tend to be more energy-efficient compared to conventional methods. The quick freezing reduces the overall processing time, leading to energy savings in the long run.
- Customization of Freezing Conditions: Liquid nitrogen freezing systems offer greater control over the freezing process. Resultingly, manufacturers can customize the conditions and tailor them to the specific requirements of different food products.
- Reduced Ice Crystal Formation: Large ice crystals can negatively impact the quality of frozen foods. However, the rapid nature of the liquid nitrogen freezing technique minimizes the formation of ice crystals. This is particularly useful for items like ice cream and frozen desserts.
- Safe Handling: Like its gaseous state, liquid nitrogen is inert and prevents unwanted flavors or chemicals from entering the food. While proper handling and storage precautions should be taken due to its extremely low temperature, when used correctly, LN2 is considered safe for food applications.
All in all, liquid nitrogen freezing offers a technologically advanced and efficient method for preserving the quality of food products as it preserves freshness and extends the shelf life across an extensive range of foods.
Other Industries that Use Liquid Nitrogen
Liquid nitrogen can be used to freeze or quickly cool water, living tissue, or other materials, making it a key element in a number of processes that demand extreme cooling or freezing methods. For example:
- Doctors use LN2 for cryosurgery to eliminate skin lesions, warts, or moles.
- Liquid nitrogen is used for the storage and transportation of blood, body parts, and foods.
- It is used in food packaging to remove oxygen and prevent oxidization of the product.
- LN2 is used in bottling plants to remove oxygen from the headspace of bottles before capping.
- Scientists use liquid nitrogen to quickly cool experiments or when cooling CCD cameras for astronomy.
- It is used for precision fitting in industry to temporarily shrink metal parts.
- It is used to freeze plastics and scrap rubber so it can be ground up efficiently for recycling.
What are the Two Main Hazards to Consider When Working With Liquid Nitrogen?
Although non-toxic, liquid nitrogen does have two major hazardous properties that are considered to be life-threatening. Due to the fact liquid nitrogen can evaporate suddenly, it can effectively displace oxygen in the air, which creates an atmosphere that is unable to support life. In addition, it can also cause severe bodily injury due to the intensity of the liquid’s extreme cold temperature.
Hazards When Working With Liquid Nitrogen Include:
- Asphyxiation
- Explosion
- Extreme cold
- Liquid spills
- Oxygen enrichment
- Pressure buildup
- Rapid phase change
What is the Expansion Rate of Nitrogen?
When it vaporizes, liquid nitrogen expands 696 times in volume and offers nothing in the way of warning properties such as odor or color. If vaporized in sufficient amounts, liquid nitrogen can reduce the atmosphere’s oxygen percentage to below 19.5%. When this occurs, there is a risk of oxygen deficiency, which can cause unconsciousness.
Fully comprehending the hazards that are affiliated with the expansion rate of nitrogen is key to avoiding accidents and ensuring worker safety. The main hazard related to the rapid expansion rate of nitrogen is associated with pressure changes that can occur when nitrogen is stored in a high-pressure vessel and/or when there is an unexpected release of compressed nitrogen gas.
By complying with the proper nitrogen safety guidelines and being aware of any associated hazards, such as the nitrogen expansion rate, workers can minimize the risks associated with handling this gas in industrial settings.
Is Liquid Nitrogen Flammable?
Liquid nitrogen – similar to nitrogen gas – is not flammable. However, as liquid nitrogen is exposed to normal temperatures and becomes a gas, it expands at a rate of 1:694.
This has led to the idea that LN could potentially cause an explosion. While technically not true, a rapid expansion of the liquid to gas as a result of a leak or a fire surrounding the LN container or transport pipes can create extremely dangerous pressures, resulting in a non-flammable explosion of the container.
Is Liquid Nitrogen Dangerous?
As mentioned, there are two primary hazards associated with liquid nitrogen. The first is asphyxiation, as its rapid expansion can swiftly displace oxygen in enclosed spaces. The second danger arises from its extremely cold temperatures, which can cause immediate freezing of exposed skin.
What Are the Safety Rules for Liquid Nitrogen?
1. Do Not Inhale Liquid Nitrogen
Asphyxiation is the main risk. A person suddenly exposed to increased levels of nitrogen gas should be promptly moved away from the source of the gas, and rescue breathing should be administered if required. If rescuers or other people working in enclosed areas are at risk of LN exposure, a self-contained breathing apparatus should be worn.
2. Wear Appropriate Personal Protective Equipment (PPE)
Worker safety depends on knowing how to properly handle and store LN. As skin exposure to liquid nitrogen can cause burns akin to frostbite, a face shield that protects the eyes and face should be used to safeguard against splashes.
Insulated gloves, aprons, and appropriate footwear covering designed for the handling of cryogenic gases should be worn to limit any contact with accidental splashes. When working with LN in confined spaces, a positive pressure, full face, air-supplied breathing apparatus should always be used.
3. Never Consume Liquid Nitrogen Directly
Direct consumption of liquid nitrogen runs the risk of severely burning your mouth and esophagus. LN2 has a very low boiling point of -196 °C, and any form of ingestion could cause asphyxiation and airway or gastric perforations.
4. Use Liquid Nitrogen in Well-Ventilated Areas
Always work in well-ventilated spaces when handling or using liquid nitrogen, and never dispose of it by pouring it directly onto the floor or pavement. Using liquid nitrogen in a confined or enclosed space can cause asphyxiation or suffocation due to oxygen displacement.
5. Only Use Containers and Equipment Designed for Cryogenic Service
Containers such as dewars ensure that the contents are held in their cryogenic state and guarantee safety in applications that involve storing or transporting cryogenic gases.
6. Read and Understand the OSHA LN Safety Guidelines
The standards for workplace safety and for anyone working around liquid nitrogen or other cryogenic gases are detailed in the Occupational Safety Health Administration OSHA Standards number 1910.101, 1910.1200 and 1910.1450.
Employers or employees should refer to both this OSHA Quick Fact Sheet as well as the published interpretation of the standard for the most current OSHA information.
While OSHA does not offer standard signage for liquid nitrogen handling, many safety sign companies can supply yellow caution signage with the text “CAUTION - Liquid Nitrogen - Gloves and Face Shield Required.”
For additional safety resources on liquid nitrogen, download the USDA guide.
Liquid Nitrogen Material Safety Data Sheet
Linde, a supplier of liquid gases in the US, offers a downloadable PDF material data safety sheet.
Liquid Nitrogen NFPA Rating
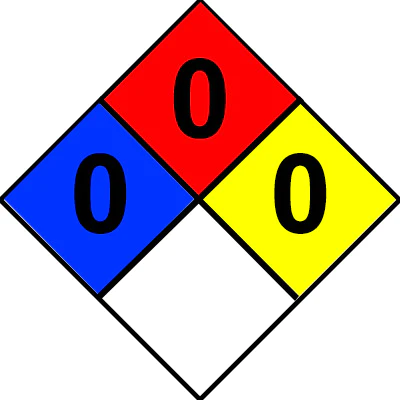
Image Credit: CO2Meter
The National Fire Protection Association NFPA 704 Rating diamond for liquid nitrogen is:
- 0 (no hazard) health
- 0 (will not burn) flammability
- 0 (stable) toxicity
- Blank for specific hazards
Nitrogen Safety Alarms
When monitoring atmospheric nitrogen levels, it is easier to monitor the oxygen molecules rather than the specific nitrogen molecules.
This is for two main reasons: a mass spectrometer would be required to accurately detect the nitrogen molecule in the atmosphere, and because nitrogen makes up 78% of the overall atmosphere is 78% any changes would be extremely difficult to detect.
Resultingly, to limit the danger of asphyxiation in enclosed areas when handling or storing liquid nitrogen, or any other cryogenic gas, oxygen depletion safety alarms should be installed.
Oxygen depletion alarms have been developed to specifically measure and alert before the oxygen concentration in an enclosed space poses a real threat to human life. Such devices safeguard employees by providing an adequate warning before entering an enclosed area where the oxygen level may have fallen below the OSHA standard of 19.5%.
For example, the Oxygen Deficiency Alarm for Low Temperatures has been specifically developed to protect employees and customers who work in close proximity to inert gases stored indoors, including nitrogen, argon, or helium. This alarm meets all OSHA requirements for safety.
For industries that use liquid nitrogen in frozen food applications or industrial settings, installing an industrial gas safety monitoring system, such as the CM-902 Industrial O2 Gas Detector, is the near-perfect solution. This device meets the strict codes that both the OSHA and the FDA apply and extends to industrial stainless steel enclosures to meet any sanitation requirements.

Image Credit: CO2Meter
The CM-902 contains a zirconium dioxide oxygen sensor. This sensor means oxygen deficiency can be measured at extremely low temperatures (down to -50 ºC). It was designed to protect individuals and employees who are likely to come in close contact with gases like nitrogen, argon, propane, or helium in confined spaces.
Due to its durability and robust enclosure, the device also is also well-suited for wash-down applications.
Portable Nitrogen Safety Devices

Image Credit: CO2Meter
For workers who move in and out of hazardous environments where liquid nitrogen is stored, used, or produced, a portable handheld safety monitor is not only advised but also considered crucial.
These devices are primarily designed for use in enclosed areas where oxygen depletion may cause personal harm. The monitor uses audible, visual, and vibrating alarms that alert personnel should oxygen levels fall below the OSHA compressed gas standards. Moreover, the portable device’s battery stores 72+ hours of charge, perfect for “on the go” workers.

This information has been sourced, reviewed and adapted from materials provided by CO2Meter, Inc.
For more information on this source, please visit CO2Meter, Inc.