Mar 26 2010
OrSense Ltd. has received approval for its low-signal, non-invasive oximetry sensor NBM-200MP from the U.S. Food and Drug Administration (FDA). OrSense develops non-invasive measurement monitors for various blood parameters.
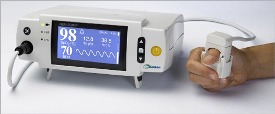 OrSense’s NMB-200MP
OrSense’s NMB-200MP
Unlike pulse oximetry which can give incorrect readings of oxygen saturation, the NBM-200MP system offers precise, continuous and non-invasive oxygen saturation measurement in conditions, such as vasoconstriction, hypothermia and hypovolemia among others. The performance of the product was validated by desaturation and ICU patient monitoring trials conducted in Europe and in the U.S.
The Head of the Intensive Care Unit of Rabin Medical Center located at Petah Tikva, Israel, Pierre Singer stated that it is essential to get the precise measurement of oxygen saturation in acute care patients and for this purpose, the non-invasive oximeter from OrSense can be effectively used for getting safe and precise oxygen saturation evaluation.
OrSense’s CEO, Lior Ma'ayan, expressed his excitement upon receiving the FDA approval and stated that the approval was a crucial element to the company’s business strategy, following the procurement of third-party integration agreements and initial clients in Europe for the company’s non-invasive multi-parameter sensor meant for low-signal oximetry and hemoglobin measurements.
The Chairman of the Board at OrSense, Shimon Eckhouse, opined that the company’s next strategy involves commercializing the technology for various non-invasive measurements of different blood parameters.