Feb 9 2018
Recurrent or metastatic tumors are responsible for most cancer deaths. While traditional treatments target rapidly dividing tumor cells, they fail to remove the highly chemoresistant tumor-initiating cells (TICs). TICs eventually lead to relapse and cause the tumors to spread to other parts of the body.
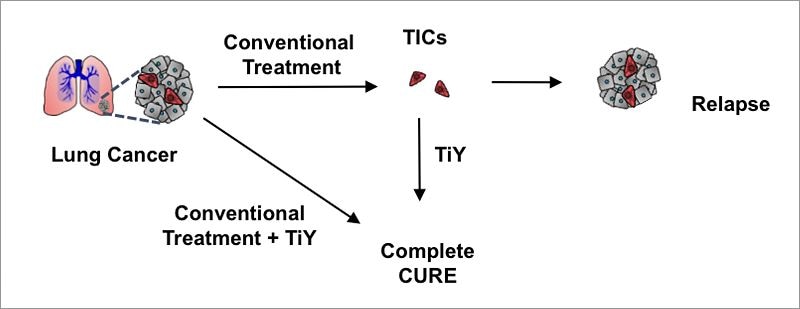 Tumor-initiating cells (TICs, in red) are resistant to conventional chemo and radiation therapies. They can repopulate the tumor (grey) causing relapse and metastasis. The scientists have developed TiY, a fluorescent sensor with drug-like properties that target TICs specifically. It could be used to visualize and suppress TICs within the tissue without the need of biopsy. (image credit: IBS)
Tumor-initiating cells (TICs, in red) are resistant to conventional chemo and radiation therapies. They can repopulate the tumor (grey) causing relapse and metastasis. The scientists have developed TiY, a fluorescent sensor with drug-like properties that target TICs specifically. It could be used to visualize and suppress TICs within the tissue without the need of biopsy. (image credit: IBS)
Now, researchers at the Center for Self-assembly and Complexity, within the Institute for Basic Science (IBS), have designed the first fluorescent sensor that enables them to visualize the TICs. The sensor is functional in the central nervous system, lung, melanoma, renal, breast, colon, ovarian, and prostate cancer cell cultures, and could well become a viable tool for anti-TIC drug development and biopsy-free post-treatment assessment.
The research was performed in POSTECH (Pohang, South Korea), in association with the Agency for Science Technology and Research (A*STAR, Singapore), and is reported in Angewandte Chemie International Edition.
In some cases, a small number of cells known as cancer stem cells, or TICs, are responsible for repopulating the tumor following therapy. With their stem cell-like properties, these cells can preserve a pool of cancer stem cells inside the tumor, and also generate new mature tumor cells. While there are specific TIC-targeting antibodies, no single universal antibody is available that can cover all types of TICs from various tissues.
By screening a countless number of fluorescent chemicals, the researchers developed a unique sensor called TiY that selectively stained the TICs of non-small cell lung cancer – a disease responsible for about 85% of all lung cancers. The researchers discovered that the TiY sensor could distinguish TICs from non-TICs in patient-derived lung tumors and various human lung cancer cell lines.
In addition to lung cancer, the TiY sensor can target TICs in 28 types of human cell lines obtained from melanoma, the central nervous system, renal, breast, colon, ovarian, and prostate cancer. “TiY has the features of a universal probe, applicable to various kinds of cancer regardless of the origin of the tissue,” explains Chang Young-Tae.
The team found that the sensor adheres to an intracellular protein known as vimentin, which is part of the cytoskeleton and gives structure and flexibility to the cell. However, vimentin is also a marker of epithelial-mesenchymal transition (EMT), which is believed to be a critical event for metastasis in epithelial tumors. The EMT procedure enables cells to adopt an invasive and migratory behavior after detaching from their neighbors. This happens to be a usual process in embryogenesis to develop new tissues, but when it takes place in cancer cells, it creates metastasis. While vimentin is a well-established TIC biomarker and with anti-vimentin antibodies available, it cannot enter the living cells, and hence cannot be used for detecting TICs in real time. The TiY, on the other hand, is a drug-like tiny molecule that has a unique characteristic of identifying TICs in vivo without biopsy and without having to isolate viable TICs for additional studies.
In the laboratory, TICs can grow as sphere-like structures, which can be easily detected under the microscope. The scientists found that vimentin directly influences this sphere-forming ability as higher concentrations of TiY led to the inhibition of sphere formation. The team also compared TiY with withaferin A (WFA) – a known vimentin-inhibitor – and found that TiY has a stronger selectivity towards TICs than WFA, as opposed to toxicity to non-TIC cells and healthy cells.
Further experiments also revealed that in mice xenograft model, TiY could suppress the tumor growth. At present, studies for detailed application of TiY to a broader range of cancer cases are ongoing along with screenings of anti-TIC drug platforms.