Image Credit: Pusan National University
Most existing techniques to detect nitroaromatic compounds cannot be used in practical situations. For example, costly laboratory equipment, like gas chromatography or X-ray imaging, cannot be deployed on the field. Hence, lately, portable and cost-effective fluorescence based chemical detectors have gained popularity.
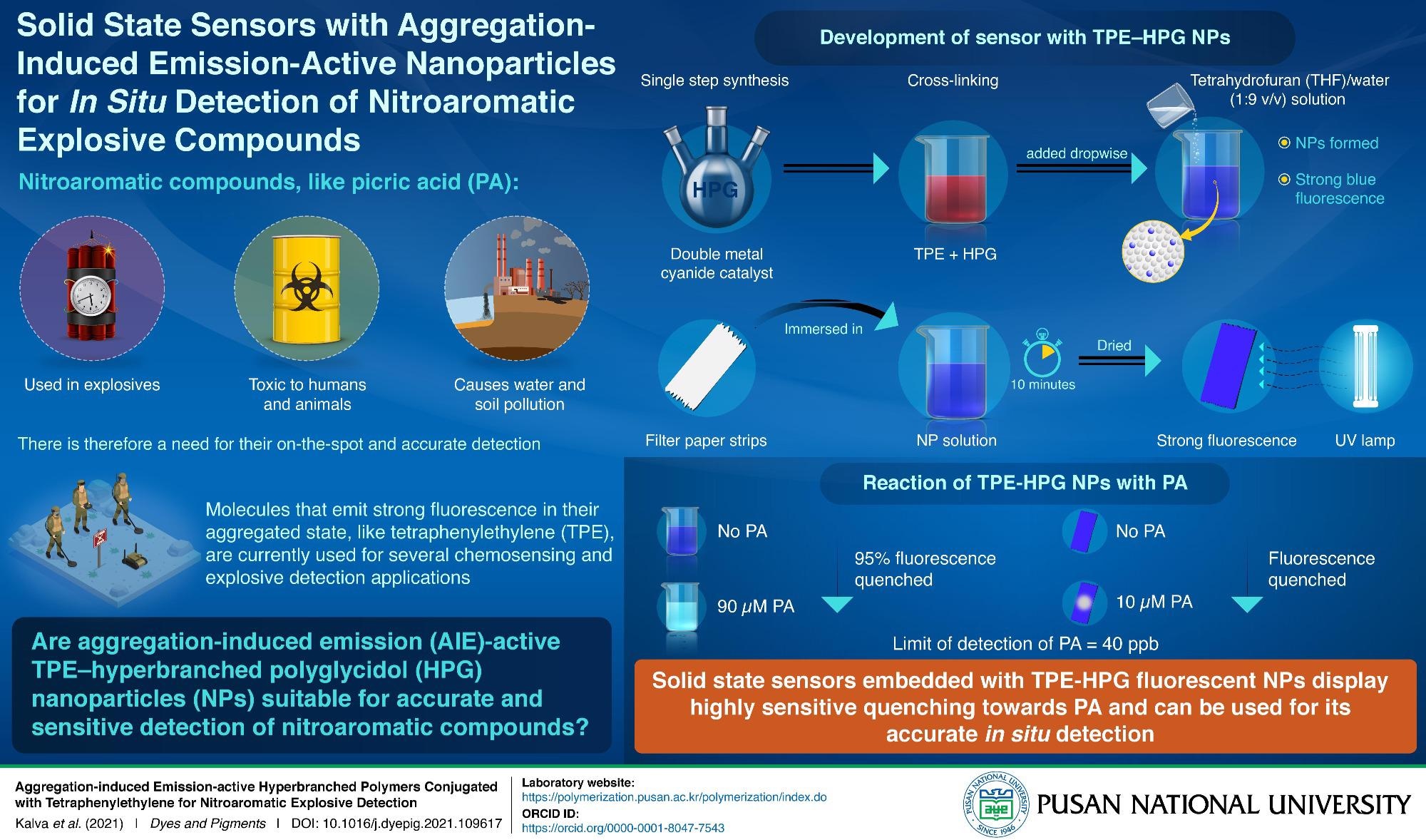
Fluorescence-based detectors operate on a phenomenon called aggregation-induced emission (AIE), which indicates that the constituent polymers emit strong fluorescence at high concentrations, under aggregated condition. This fluorescence shows quenching (reduction in intensity) behavior in contact with nitroaromatic compounds. Chemicals that are often used to formulate AIE polymers are tetraphenylethylene (TPE), thriphenylamine (TPA), and distyrylanthracene derivatives. Conventional methods available for the formulation of AIE polymers are highly complicated, involving multi-step purification processes, proving detrimental in the application of AIE polymer-based probes. Therefore, simplification of their chemical synthesis is the need of the hour.
Fortunately, a group of researchers from Pusan National University, Republic of Korea, led by Prof. Il Kim, were successful in developing a single-step synthesis procedure for an AIE active polymer—TPE conjugated-hyperbranched polyglycidol (HPG).
We synthesized AIE active nanoparticles using commercially available HPG, that too in a simple single-step process. This new protocol will eliminate earlier difficulties faced in the synthesis of AIE active nanoparticles and promote their use in the detection of nitroaromatic compounds.
Il Kim, Professor, School of Chemical Engineering, Pusan National University
The novel single step protocol produces a TPE-HPG polymer solution that is added to water for formation of bright blue fluorescent TPE-HPG aggregated nanoparticles. Through empirical analysis, the researchers observed that the strong blue fluorescence of these nanoparticles is quenched by almost 95% on addition of 90 µM concentration of PA. The minimum concentration of PA that can be detected using TPE-HPG nanoparticles is 40 parts per billion.
As solid-state sensors are more convenient for on-field applications than nanoparticle solutions, the team immobilized the TPE-HPG nanoparticles on strips of paper by following a simple dip and dry process. The strips of paper were dipped in nanoparticle solution for 10 minutes followed by drying under vacuum at 50 ᵒC for 24 hours. The paper-based sensors prepared this way emitted bright blue fluorescence under UV light, which was progressively quenched on addition of higher concentrations of PA.
We demonstrated a facile synthesis protocol for a fluorescent nanoparticle that can be deployed as a solid-state sensor for detecting explosives. The full potential of this technology is as yet untapped; it can be further customized for applications in chemosensing, bioimaging, and optoelectronics.
Il Kim, Professor, School of Chemical Engineering, Pusan National University
Overall, by enabling fast, accurate, and in situ detection of explosives, the findings of this study could offer a significant technical support to security agencies worldwide.
Reference
Title of original paper: Aggregation-induced emission-active hyperbranched polymers conjugated with tetraphenylethylene for nitroaromatic explosive detection
Journal: Dyes and Pigments
DOI: https://doi.org/10.1016/j.dyepig.2021.109617
Corresponding author’s email: [email protected]
Laboratory website : https://polymerization.pusan.ac.kr/polymerization/index.do
ORCID ID: https://orcid.org/0000-0001-8047-7543
About Pusan National University
Pusan National University, located in Busan, South Korea, was founded in 1946, and is now the no. 1 national university of South Korea in research and educational competency. The multi-campus university also has other smaller campuses in Yangsan, Miryang, and Ami. The university prides itself on the principles of truth, freedom, and service, and has approximately 30,000 students, 1200 professors, and 750 faculty members. The university is composed of 14 colleges (schools) and one independent division, with 103 departments in all.
Website: https://www.pusan.ac.kr/eng/Main.do
About the author
Professor Il Kim is a Professor in the School of Chemical Engineering, Pusan National University (PNU). He received his PhD in Chemical Engineering from Korea Advanced Institute of Science and Engineering in 1990. In 2013, he was appointed Director of the Brain Korea (BK) 21 PLUS Center for Advanced Chemical Technology, and was also appointed as the chairman of the Department of Chemical Engineering, Polymer Science and Engineering in PNU. In 2018 he was appointed Vice President of the Korea Polymer Society. He has published 400+ peer-reviewed papers and received over 20 awards for his contribution to science and technology.