Bioinspired sensor for the endoscopic imaging system includes a pixel array that can capture light on six different spectral channels. Image Credit: George et al., doi.org/ 10.1117/1.JBO.28.5.056002
But there is a huge risk of cancer recurrence if even a few cancerous cells are left behind following surgical resection. To avoid this happening, scientists came up with fluorescence-guided surgery (FGS).
In FGS, patients are injected with a fluorescent probe that preferentially binds to tumor cells, thereby allowing surgeons to determine lesions easily using specialized endoscopes that detect the essential excitation light.
Unfortunately, tumors can be highly heterogeneous, and it is not possible to detect them all using a single fluorescent probe. Hence, one of the frontiers in FGS is making use of cocktails of several fluorescent probes (a.k.a. “tracers”) to detect an extensive range of tumors, as well as to decrease false positives and negatives.
Despite some advances in this direction, clinically approved endoscopes have all been improved to detect just a single tracer. Furthermore, multi-tracer instruments that are under development at present are huge since they need several imaging sensors and optical components.
In a study recently reported in the Journal of Biomedical Optics (JBO), a research group from the University of Illinois Urbana–Champaign reported a novel endoscopic imaging system whose design could greatly expedite the multi-tracer FGS’ adoption.
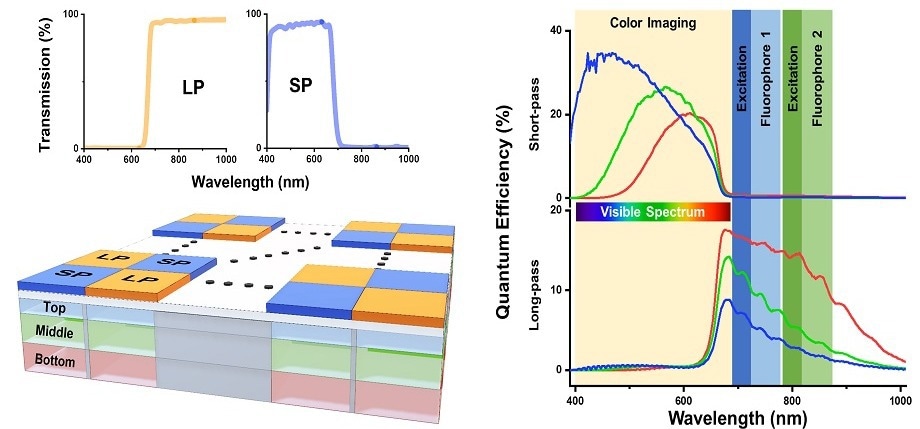
At the core of this design rests a ground-breaking hexa-chromatic bioinspired imaging sensor (BIS), which the scientists modeled depending on the visual system of the mantis shrimp.
The sensor comprises three layers of vertically stacked photodetectors that have been covered with a checkerboard-like arrangement of two different filters; one filters visible light, and the other filters near-infrared (NIR) light.
The outcome is a single-chip camera that has the potential to efficiently capture light on six different spectral channels. This helps it detect even the highly delicate variations in fluorescence emission from the tissue that is being imaged.
To put its performance into perspective, this BIS can distinguish fluorescent tracers with emission peaks that are only 20 (nm) aside. Such a feat is not feasible with clinically approved imaging instruments available currently.
To make use of the BIS efficiently, the scientists also had to design a suitable excitation light source for triggering the fluorescent tracers. As a result, they utilized custom bifurcated optical fibers linked to three independent light sources—a white LED and two NIR lasers at 665 and 785 nm.
The scientists linked the integrated output of the fibers at the beginning of the rigid endoscope. In this way, by utilizing a single imaging sensor and a single light input, they made the device less heavy compared to other multi-tracer imaging systems.
Characterization and benchmarking tests were performed by the scientists to identify the device’s spatial resolution and sensitivity. They executed in vivo experiments on a mouse model for breast cancer.
These mice were injected with the help of a 680 nm tracer, an 800 nm tracer, or an equal mixture of the two. The suggested system could clearly distinguish between the fluorescence signatures that have been produced by the individual tracers and also the mixture.
Aiming to show the clinical potential of their endoscope, the scientists utilized it to image lung cancer nodules that had been just eliminated from patients. Even though these patients had only been injected with a single fluorescent tracer, the suggested device was still capable of precisely differentiating malignant nodules from healthy tissue.
On the whole, significant engineering breakthroughs were achieved by the scientists that will set the stage for the adoption of multi-tracer FGS. As a result of its higher spatial resolution and its extraordinary capacity to detect small changes in fluorescence emission, the suggested endoscopic imaging system will assist doctors in detecting smaller or differently hidden tumors more simply.
Hopefully, this will enhance the long-term survival of patients with operable cancer.
Journal Reference
George, M. B., et al. (2023) Bioinspired color-near infrared endoscopic imaging system for molecular guided cancer surgery. Journal of Biomedical Optics. doi.org/10.1117/1.JBO.28.5.056002.