Abbott, a medical devices and pharmaceutical technology developer, has announced that the latest addition to its glucose monitoring systems. The FreeStyle InsuLinx Blood Glucose Monitoring System has received clearance from the US Food and Drug Administration.
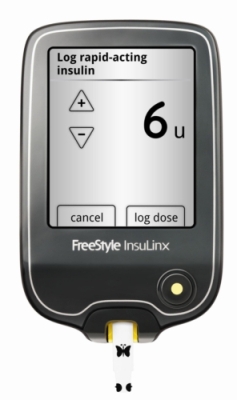 FreeStyle InsuLinx blood glucose monitor
FreeStyle InsuLinx blood glucose monitor
Featuring a touch-screen interface, personalized features and an automated logbook, the blood glucose monitoring system has been developed to improve care for diabetes patients.
The American Diabetes Association reports that nearly 25.8 million people in the USA have diabetes and a large percentage of the diabetic population requires insulin to manage the disease. The FreeStyle technology allows patients dependent on insulin to observe their blood glucose levels and manage their condition more effectively. The new device is available with the FreeStyle Auto-Assist software that allows patients to track their progress, analyze the latest trends and display the recorded data to their healthcare providers.
The FreeStyle InsuLinx Blood Glucose Monitoring System features an easy-to-use touch screen, an automated logbook to log insulin doses and the level of blood glucose, meal markers and the FreeStyle software that allows the system to be connected to a computer to manage reports, messages and reminders. The device is compatible with Abbott’s FreeStyle InsuLinx test strips for blood glucose.
The Senior Vice President of Abbott Diabetes Care, Heather L Mason stated that the InsuLinx Blood Glucose Monitoring system offers data sharing tools, improved functionality and personalization features to improve the patient’s diabetes management experience.
The blood glucose monitoring systems offered by Abbott Diabetes Care also include the FreeStyle Lite Blood Glucose Monitoring System, the FreeStyle Freedom Lite Blood Glucose Monitoring System and the Precision Xtra Blood Glucose and ß-Ketone Monitoring System.