Measuring electrical conductivity in liquid substances is a highly powerful diagnostic and analytical tool in a range of application in spite of its simplicity.
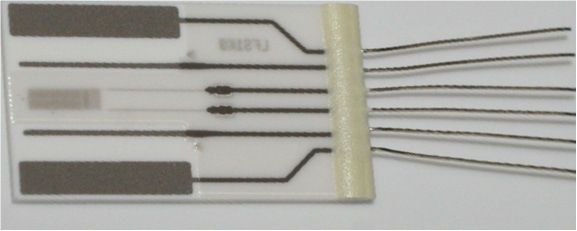
Figure 1.
The latest thin-film conductivity sensor element (figure 1, above) is a suitable alternative to the bulky, classical conductivity sensors of the past.
Theoretical Background
An electrolyte is a liquid that contains ions. The ions behave as charge carriers and a current flows when a voltage is applied. Hence the liquid quality can be assessed by determining the conductivity. The liquid conductivity is based on two temperature-dependent parameters that include ion concentration and their mobility. A temperature sensor is placed directly at the measurement point for improved accuracy.
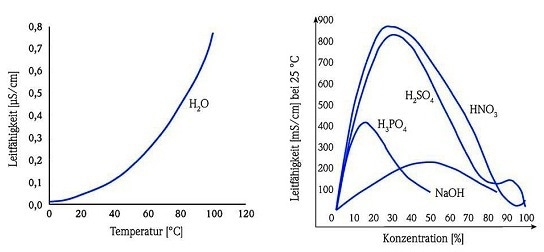
Figure 2. Liquid conductivity based on two temperature-dependent parameters.
Selected Conductivity of Electrolytes
The electrical conductivity of electrolytes is provided in the table below:
| Electrolyte |
Electrical conductivity |
| pS/cm |
S/m |
| Ultra pure water |
0.05-0.1 |
5-10*10-6 |
| Tap water |
300-800 |
0.03-0.08 |
| NaCl (0.2 g/l) |
4'000 |
0.4 |
| NaCl (2 g/l) |
38'600 |
3.86 |
| Seawater |
~56'000 |
~5.6 |
| Bulk silver (for comparison) |
62.5*106 |
6'250 |
Measurement Principles
The conductivity (using electrodes) is shown in Figure 3.
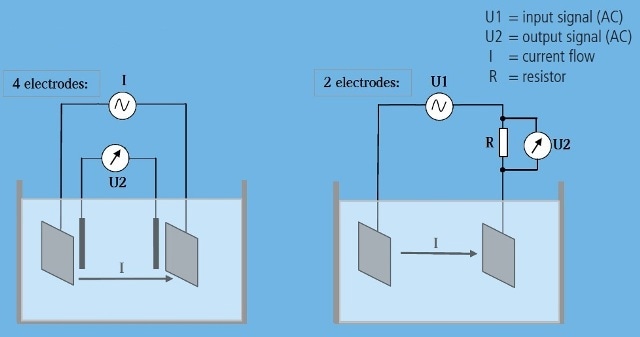
Figure 3. Measurement Principles.
In order to minimize degradation of the electrolyte and the electrode, AC excitation is recommended.
Conductivity and Cell Constant
The conductivity value which is obtained as a result of the measurement additionally depends on the cell geometry. The impact of the cell geometry can be eliminated by introducing the so-called cell constant. The electrical conductivity κ using the following formula can be obtained at a particular temperature:

The exact cell constant value can be obtained because of calibration measurements in standard solutions. In order to avoid additional measurement errors, it is essential to use a solution having electrical conductivity values close to the values of the intended application solution.
Conductivity

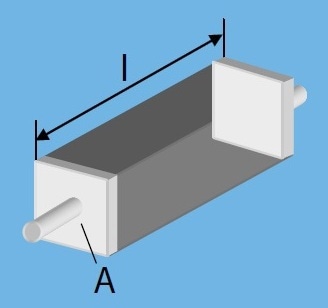
Cell constant is influenced by:
- Boundary effects
- Planar geometry of chip layout
where κ is the electrical conductivity, l is the length and A is the area.
Basic Layout: 4 Linear Electrodes
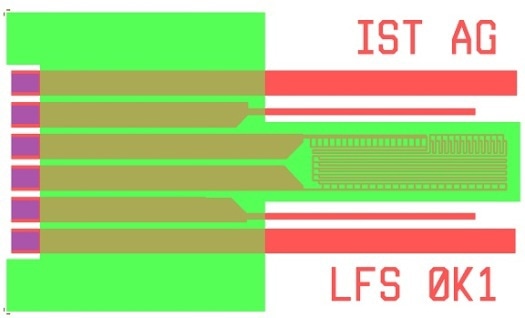
Major Parameters for Customer Specific Designs
The key parameters for customer specific designs are:
- Measurement liquid characteristics (stability of platinum electrodes): Customer testing is mostly required and samples can be provided.
- Measurement range(cell constant) can be adjusted by the electrode geometry.
- Read out value is AC 300 to 3000 Hz, 1.6 VPP or less.
- The assembly method is encapsulated wires and fixation.
- Customer expectations include only chip, assembly and electrical readout (under development)

This information has been sourced, reviewed and adapted from materials provided by Innovative Sensor Technology.
For more information on this source, please visit Innovative Sensor Technology.