Hydrogen is an essential industrial gas that is also becoming increasingly important as a fuel. Hydrogen, however, is fundamentally different from any other fuel source, in terms of both its hazards and its function.1

Image Credit: International Gas Detectors Ltd
As the global hydrogen economy continues to grow, it is anticipated that hydrogen will play a key role in decarbonizing the world’s energy supplies. The widespread adoption of hydrogen technologies necessitates the use of robust safety measures, including the deployment of dependable hydrogen gas detection systems.
The Importance of Hydrogen
Almost every fuel currently in use worldwide is derived from naturally occurring gas or oil. These fuels are a finite resource and their ongoing use is damaging to the environment. Because of this, the world is moving more towards renewable energy sources.
Increased renewable energy production poses its own challenges, however. There are questions around how best to store energy produced by intermittent sources such as solar and wind as well as other issues around the capacity of transport and industrial sectors currently reliant on energy-dense chemical fuels to operate in a post-carbon economy.
Hydrogen presents a viable solution to both of these issues. Hydrogen is commonly used as an industrial gas and is currently produced via steam methane reforming (SMR).
Renewable energy can be employed in the creation of hydrogen gas via processes like water electrolysis, essentially locking that energy within hydrogen molecules. In this context, hydrogen acts as an energy storage medium as opposed to conventional fuel.
Hydrogen has a number of advantages over other energy storage technologies. Like traditional fuels, hydrogen displays a very high energy density. This energy density is far higher than that of batteries or capacitors while being capable of storing energy indefinitely with no losses.
Unlike traditional fuels, hydrogen does not produce dangerous emissions at the point of use. Water remains the only by-product of burning hydrogen. A Proton Exchange Membrane (PEM) cell uses hydrogen gas and oxygen gas as fuel, effectively converting chemical potential energy into electrical energy.
While hydrogen technology remains relatively new, there is already evidence to suggest that hydrogen may become considerably less expensive per unit of energy stored than batteries.1,2,3
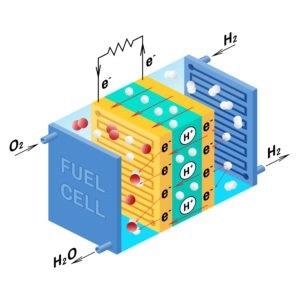
Fuel cell diagram. Vector. Device that converts chemical potential energy into electrical energy. A PEM, Proton Exchange Membrane cell uses hydrogen gas and oxygen gas as fuel. Image Credit: International Gas Detectors Ltd
This potential has led to the rapid growth of the hydrogen economy, with the global hydrogen market set to increase by approximately 6% per year, growing to $155 billion by 2022.4
The Risks of Hydrogen
The increased uptake of hydrogen technologies also presents a number of risks. Hydrogen performs well as a fuel, but it also provides a much greater risk of explosion compared to other gas and liquid fuels.
Hydrogen is much harder to contain than other gases. Hydrogen gas is made up of H2 molecules, with each molecule comprised of two hydrogen atoms bound together. H2 is the smallest molecule in the universe and is extremely prone to leaking out of containment.5
Hydrogen is also highly flammable. Hydrogen in air is flammable at concentrations between 4% and 75% by volume. In contrast, methane is flammable in air in concentrations between 4.4% and 17% by volume.
The energy necessary for the ignition of a hydrogen/air mixture is also significantly lower than that of other fuels. The minimum amount of energy needed to ignite a hydrogen/air mixture is just 0.017 mJ. The minimum ignition energy for hydrocarbon fuel gases is comparatively much higher, at around 0.3 mJ for propane/air or methane/air mixtures.6,7
These factors mean that hydrogen leaks are common, and even miniscule hydrogen leaks can ignite relatively easily.8 As such, suitable measures must be put in place to manage and minimize any risk of hydrogen leaks.
Hydrogen Gas Detection Technology
Hydrogen is a colorless, odorless, and tasteless gas, meaning that industry must rely on hydrogen gas detectors to detect leaks. IGD offers two technologies that are appropriate for hydrogen detection: pellistor sensors and electrochemical sensors.
Pellistor
Pellistor (also known as a catalytic bead) sensors employ a catalyst that prompts flammable gas entering the sensor to ignite at a far lower temperature than usual. As combustion occurs, a proportional amount of heat is produced to match the amount of flammable gas present.
This measurement can be used to determine the concentration of flammable gases present, and this value can be expressed as a percentage of the lower explosive limit (%LEL).

Pictured: TOC-750 safe area addressable gas detector. Image Credit: International Gas Detectors Ltd
Pellistor sensors are generally utilized as a generic ‘catch-all’ flammable gas detection technology. Pellistor sensors will respond to any flammable gas, measuring 0-100% LEL. As a 4% concentration of hydrogen is potentially explosive, this would correspond to 100% LEL.
Legislation like the UK Dangerous Substance Explosive Atmosphere Regulations (DSEAR) typically requires that an atmosphere is kept below 25% LEL.
Pellistor gas detectors from IGD are the most robust, reliable, and cost-effective detectors of their kind, making them a popular and reliable choice for hydrogen gas detection.
Electrochemical
Electrochemical sensors react to the target gas (for example, hydrogen) using an electrolyte, which will generate a current that is proportional to the amount of gas detected. This method enables a more sensitive hydrogen gas detection than that of pellistor sensors.
For example, 25% LEL would correspond to 1% hydrogen concentration or 10000 ppm. IGD electrochemical gas detectors offer gas sensitivity in the ranges of 0-1000 ppm to 0-40000 ppm.
The disadvantage of this acute sensitivity is that electrochemical sensors are prone to damage or destruction if they are exposed to target gas levels exceeding their measurement range. When this happens, sensors need to be replaced immediately to ensure continued monitoring.
IGD electrochemical hydrogen detectors are well suited to applications where it is necessary to detect hydrogen at low levels.
Other Detection Technologies
A number of other gas detection technologies exist, but they are not recommended for the detection of hydrogen.
For example, infrared sensors cannot detect hydrogen as diatomic molecules like hydrogen do not absorb infrared radiation.
Semiconductor gas detectors may be employed in hydrogen detection, but these sensors will also respond to a wide range of other gases and vapors. Semiconductor sensors are therefore not advisable for use in hydrogen detection applications due to the likelihood of false alarms.
Another viable technology is thermal conductivity, but its low sensitivity and selectivity render this approach a poor choice for hydrogen detection applications.
Hydrogen Gas Detection Solutions from IGD
Fixed Detection Systems
International Gas Detectors offer a diverse array of fixed electrochemical and pellistor hydrogen gas detection systems. These solutions are designed to accommodate all application requirements.
The IP54 addressable 750 safe area detectors can be used for non-ATEX applications, while IP68 750X ATEX/IECEx range is explosion proof. TOC-750S addressable aspirated detectors are an ideal solution for cleanrooms or hard to access areas, offering clients a reliable digital gas detection system which is Internet of Things compatible.
The TOCSIN 903 ATEX/IECEx standalone non-intrusive gas detectors are also a good option for customers looking for a robust hydrogen gas detection system.

Image Credit: International Gas Detectors Ltd
Each of these systems boasts a robust selection of features, benefits and cost saving technologies that are not available in other hydrogen gas detection systems.
Portable Hydrogen Gas Detectors
International Gas Detectors also provide portable hydrogen monitors for enhanced leak detection and personal safety. A single gas monitor, working at 0-1000 ppm, can be used to help identify leaks in equipment. Alternatively, a standard 4 gas monitor can be used when working in confined spaces.
References
- The Future of Hydrogen – Analysis. IEA https://www.iea.org/reports/the-future-of-hydrogen.
- Pellow, M. A., Emmott, C. J. M., Barnhart, C. J. & Benson, S. M. Hydrogen or batteries for grid storage? A net energy analysis. Energy Environ. Sci. 8, 1938–1952 (2015).
- Batteries and hydrogen technology: keys for a clean energy future – Analysis. IEA https://www.iea.org/articles/batteries-and-hydrogen-technology-keys-for-a-clean-energy-future.
- Bezdek, R. H. The hydrogen economy and jobs of the future. Renew. Energy Environ. Sustain. 4, 1 (2019).
- Lanz, W. Hydrogen Properties. Hydrogen Fuel 47 (2001).
- ISO – ISO/TR 15916:2015 – Basic considerations for the safety of hydrogen systems. https://www.iso.org/standard/56546.html.
- Haase, H. Electrostatic hazards. Their evaluation and control. (Verlag Chemie, 1977).
- Butler, M. S., Moran, C. W., Sunderland, P. B. & Axelbaum, R. L. Limits for hydrogen leaks that can support stable flames. International Journal of Hydrogen Energy 34, 5174–5182 (2009).

This information has been sourced, reviewed and adapted from materials provided by International Gas Detectors Ltd.
For more information on this source, please visit International Gas Detectors Ltd.