Many sectors heavily rely on tight control of the atmospheric composition.
Gas sensors like electrochemical, catalytic pellistors, thermal conductivity, metal oxide, and optical gas sensors have all been widely studied. Resistive gas sensors are popular because of their low cost and long lifespan.
To identify harmful gases, materials have been utilized, including metal oxides, conducting polymers, carbon compounds, and their composites.
Since their characteristics fluctuate quickly, recent research has shown that metal oxide composites with trace carbon could increase the performance of the final material when included in various types of sensors.
Despite this, there has been little investigation into the gas-sensing characteristics of carbon-doped TiO2. The successful properties of resistive sensors for determining ammonium gas based on carbon gel-TiO2 composites are demonstrated in this study.
Methodology
Carbon gel-TiO2 nanocomposites were synthesized and characterized by combining complementary techniques to analyze the physical and chemical properties. The sensing films were prepared at 50 μm thick.
The measurement system is depicted in Figure 1. The tests were carried out in a homemade plastic climate chamber, in which the sensor was installed and attached to the electrometer.
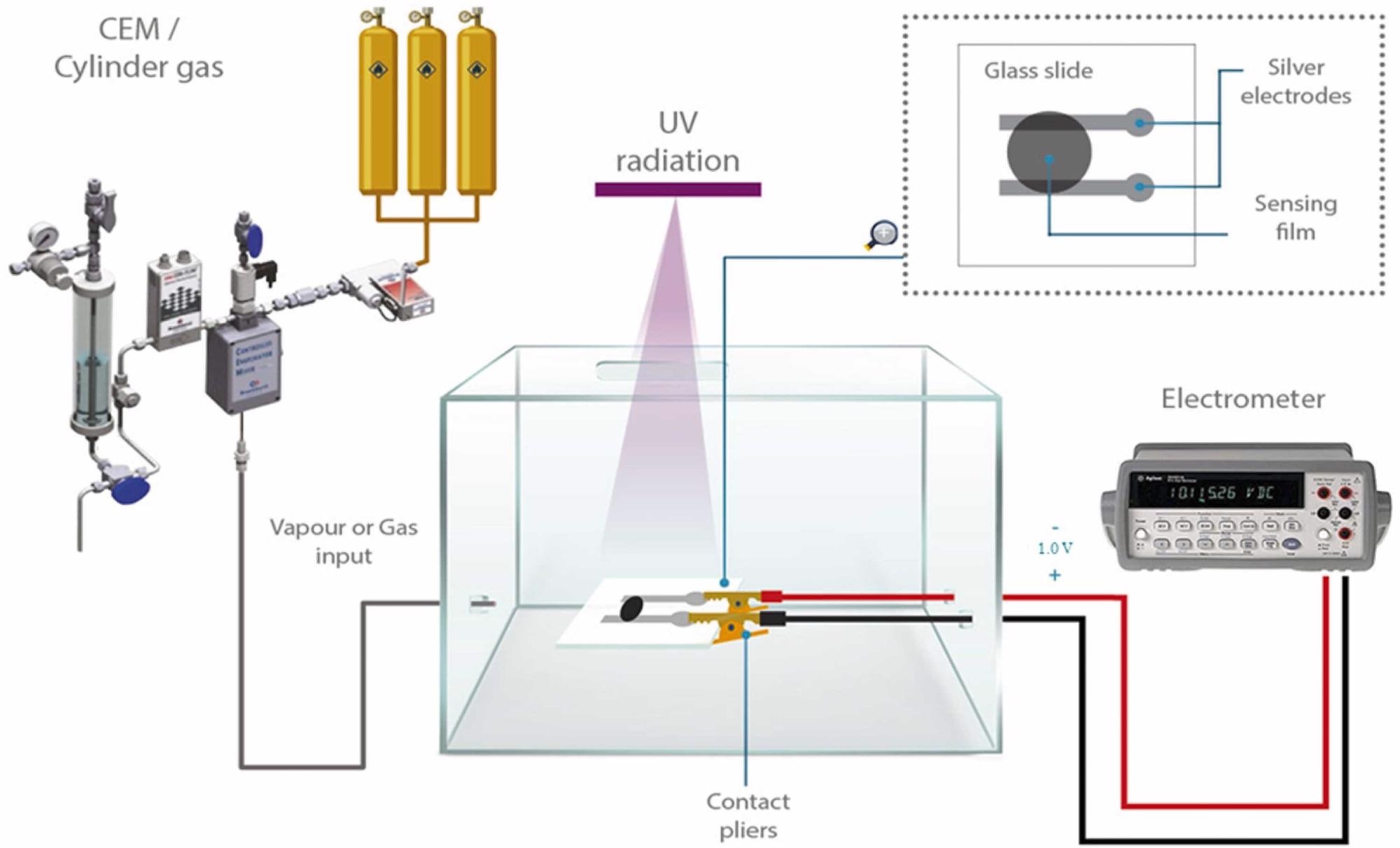
Figure 1. Schematic diagram for the homemade gas sensor testing system. Image Credit: Fernández-Ramos, et al., 2022
To rule out experimental error, the measurements were performed three times. With a continuous 1.0 V potential difference given to the electrodes and a satisfactory ohmic contact between the silver electrodes and the sensing film, the flow through the sensing film was monitored.
Results and Discussion
Four RF polymer-TiO2 composites with theoretical TiO2 content of 10, 20, 30, and 50 wt% were made. Composites lose weight when they are pyrolyzed. The weight loss (WL) is primarily linked to the transition of the organic RF-polymer into a carbon gel, which may be analyzed in three stages, matching the DTG profile’s peaks.
HRSEM was used to examine the morphology of the samples (Figure 2A). Massive, dense, and compact particles are covered by another structured phase in the shape of a 3D network of almost spherical particles that combine to produce a porous structure in general.
For RF carbon gels, this is the characteristic coral-like nanostructure described earlier. At intermediate TiO2 loading, HRTEM mapping (Figure 2B) indicates the presence of extremely tiny and uniformly distributed TiO2 nanoparticles (Figure 2Bb).
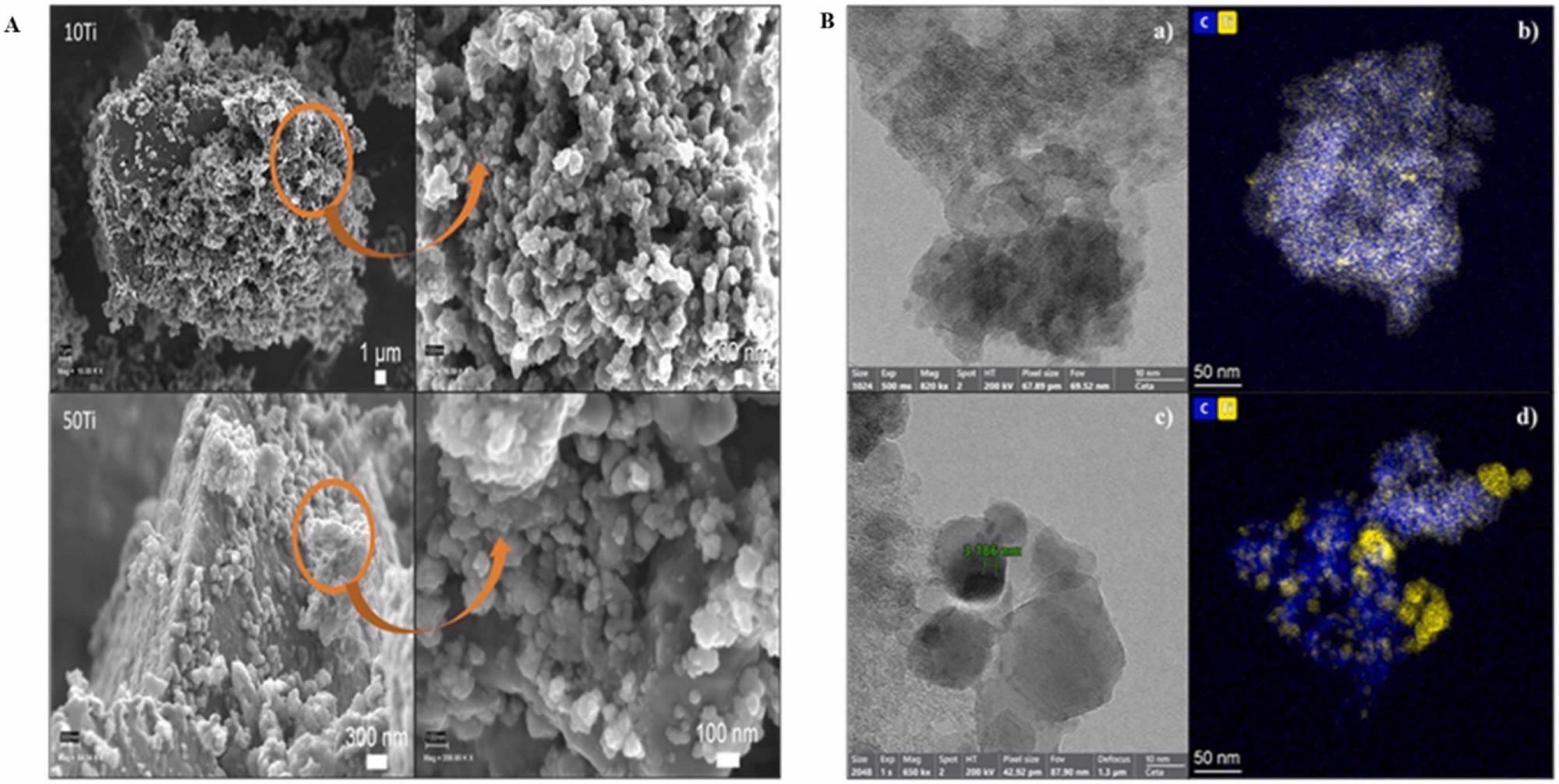
Figure 2. A: HRSEM images showing the morphology of carbon gel–TiO2 composites; B: HRTEM and mapping images of (a, b) 20Ti and (c, d) 50Ti (measurements correspond to 10 interlayer distances). Image Credit: Fernández-Ramos, et al., 2022
An in-depth examination revealed TiO2 nanoparticles with a size of typically less than 4–5 nm, but which were crystalline, as evidenced by the ordered layers inside the nanocrystals. For most TiO2 nanoparticles, the interlayer distance is roughly 3.5 Å, which correlates to the (101) crystallographic planes of anatase. With a larger TiO2 content, sintering and particle size improved (Figure 2Bc).
To understand the influence of each phase on this parameter, the electrical resistance of the composites was measured in both dark and UV-irradiation conditions before monitoring the efficiency of the sensing films against the various gases.
The sensor design was adjusted to provide the best detection sensitivity. The slope of the corresponding dependence grows as the proportion of TiO2 in the sensor increases, enhancing the sensitivity.
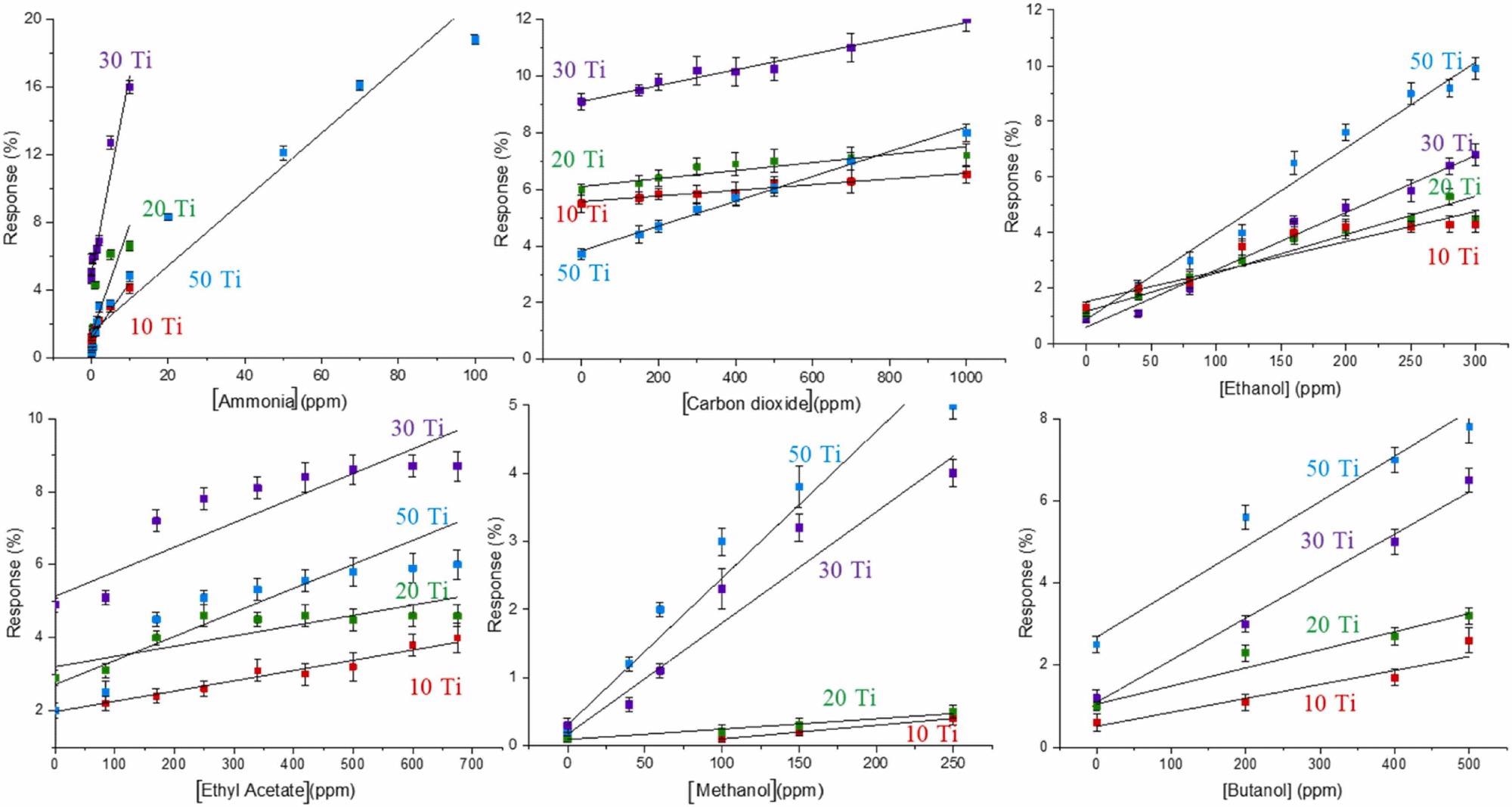
Figure 3. Response (%), sensitive response, of sensors prepared with the different carbon gel-XTiO2 composites against selected gases. Image Credit: Fernández-Ramos, et al., 2022
As the amount of TiO2 in the composite increases, the resistance lowers (Table 1), speeding up the movement of electrons on the material’s surface and therefore boosting the specific surface area and, as a result, the sensitivity to gas (Figure 4). The analytical characteristics of sensing membrane 50Ti for all gases analyzed are shown in Table 2.
Table 1. Influence of UV light on the resistance value of different materials in air. Source: Fernández-Ramos, et al., 2022
| Material |
Electrical Resistance (kΩ) |
| Dark (without UV) |
UV |
| C |
34228.3 ± 32.5 |
32725.0 ± 36.3 |
| TiO2 |
90.7 ± 17.2 |
85.2 ± 12.4 |
| P25 |
119.1 ± 8.2 |
100.4 ± 6.2 |
| 10Ti |
25540.0 ± 22.1 |
24021.3 ± 18.3 |
| 20Ti |
9236.3 ± 7.3 |
8205.1 ± 8.2 |
| 30Ti |
5432.6 ± 6.5 |
4302.1 ± 7.2 |
| 50Ti |
528.7 ± 4.6 |
430.2 ± 3.8 |
Table 2. Analytical characteristics of sensing membrane 50Ti for all gases tested. Source: Fernández-Ramos, et al., 2022
| Analytical Parameter |
Gases |
| NH3 |
C2H5OH |
CH3OH |
C4H10O |
C4H8O2 |
CO2 |
| Slope (b) |
0.33±0.029 |
0.034±0.0004 |
0.04±0.004 |
0.010±0.004 |
0.012±0.001 |
0.005±0.001 |
| Intercept (a) |
1.12±0.058 |
0.86±0.014 |
0.26±0.022 |
1.145±0.018 |
2.057±0.018 |
3.69±0.020 |
| R2 |
0.9750 |
0.9874 |
0.9950 |
0.9934 |
0.9798 |
0.9947 |
| LOD (ppm) |
1.55 |
2.55 |
2.62 |
48.00 |
46.75 |
15.60 |
| LQD (ppm) |
5.20 |
8.52 |
8.75 |
162.00 |
155.83 |
52.10 |
| Response time t90 |
50 |
20 |
25 |
32 |
30 |
28 |
| Recovery time t10 |
170 |
60 |
58 |
65 |
68 |
62 |
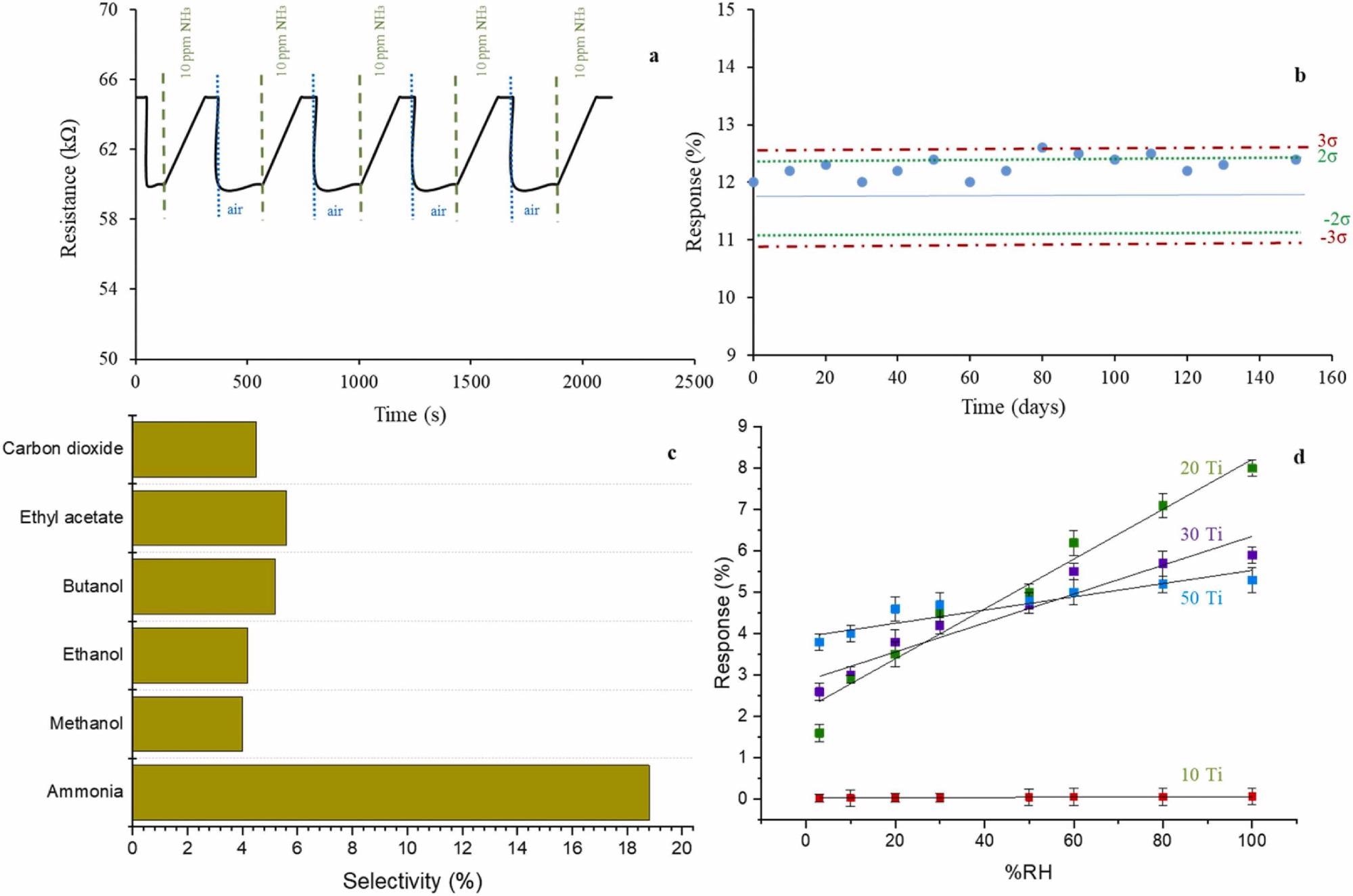
Figure 4. (a): Response and recovery curves of composite 50Ti at 10 ppm NH3; b): Shewhart control chart for checking long-term stability of the sensor; c): Selectivity of 50Ti for 100 ppm of gases.; d): Humidity influence on XTi sensing membranes. All experiments were carried out at room temperature (20 ºC). Image Credit: Fernández-Ramos, et al., 2022
The sensor test with a particular time interval during which the sensors were stored in an open-air setting verified the long-term stability.
Figure 4b displays the sensor’s control chart—the sensor signal stays within the specified specification limit after 150 days. During the testing period, the sensor showed outstanding long-term stability, indicating that it would last a long time.
For samples with an intermediate content of both phases and, thus, intermediate hydrophilicity, the slope of the lines in Figure 4d is larger. The carbon gel-TiO2 nanoparticle gas sensor has excellent moisture stability, according to the findings. All of the trials were conducted at room temperature.
Conclusion
As sensing materials, carbon gel-TiO2 nanocomposites have been presented. At room temperature and under UV irradiation, the C-XTiO2 exhibits distinct gas sensitivity to ammonia, ethanol, methanol, butanol, ethyl acetate, and carbon dioxide.
The resistive sensor works in the same way as an n-type semiconductor does. With a low applied voltage of 1.0 V, carbon-doped with 84% TiO2 has the best sensor characteristics: strong selectivity towards ammonia, low response and recovery times, consistency over time, and low humidity impact on the performance.
These initial findings, together with the low cost of carbon, make carbon doped with TiO2 sensors promising candidates for creating low-cost commercial resistive sensors for specific tracking of certain gas analytes.
Journal Reference:
Fernández-Ramos, M. D., Capitán-Vallvey, L. F., Pastrana-Martínez, L. M., Morales-Torres, S., Maldonado-Hódar, F. J. (2022) Carbon Gel-TiO2 Nanocomposites as a New Platform for Chemoresistive Gas Sensor at Room Temperature. Sensors and Actuators B: Chemical, p. 132103. Available Online: https://www.sciencedirect.com/science/article/pii/S0925400522007456.
References and Further Reading
- Paul, V & Pandey, R (2014) Role of internal atmosphere on fruit ripening and storability--a review. Journal of Food Science and Technology, 51, pp. 1223–1250. doi.org/10.1007/s13197-011-0583-x.
- Mabrook, M & Hawkins, P (2001) A rapidly-responding sensor for benzene, methanol and ethanol vapours based on films of titanium dioxide dispersed in a polymer operating at room temperature. Sensors and Actuators B: Chemical, 75, pp. 197–202. doi.org/10.1016/S0925-4005(01)00761-4.
- Yang, Z., et al. (2021) Flexible resistive NO2 gas sensor of three-dimensional crumpled MXene Ti3C2Tx/ZnO spheres for room temperature application. Sensors and Actuators B: Chemical, 326, p. 128828. doi.org/10.1016/j.snb.2020.128828.
- Hamad, H., et al. (2018) On the Interactions and Synergism between Phases of Carbon⁻Phosphorus⁻Titanium Composites Synthetized from Cellulose for the Removal of the Orange-G Dye. Materials (Basel), 11, p. 1766.
- Pastrana-Martínez, L. M., et al. (2018) Photocatalytic activity of functionalized nanodiamond-TiO2 composites towards water pollutants degradation under UV/Vis irradiation. Applied Surface Science, 458, pp. 839–848. doi.org/10.1016/j.apsusc.2018.07.102.
- Dankeaw, A., et al. (2017) In-situ one-step method for fabricating three-dimensional grass-like carbon-doped ZrO2 films for room temperature alcohol and acetone sensors. Sensors and Actuators B: Chemical, 242, pp. 202–214. doi.org/10.1016/j.snb.2016.11.055.
- Nunes Simonetti, E. A., et al. (2021) TiO2 as a gas sensor: The novel carbon structures and noble metals as new elements for enhancing sensitivity – A review. Ceramics International, 47, pp. 17844–17876. doi.org/10.1016/j.ceramint.2021.03.189.
- Bailón, E., et al. (2016) Chemoselective Pt-catalysts supported on carbon-TiO2 composites for the direct hydrogenation of citral to unsaturated alcohols. Journal of Catalysis, 344. doi.org/10.1016/j.jcat.2016.09.026.
- Smulko, J & Trawka, M (2015) Gas selectivity enhancement by sampling-and-hold method in resistive gas sensors. Sensors and Actuators B: Chemical, 219, pp. 17–21. doi.org/10.1016/j.snb.2015.04.120
- Elmouwahidi, A., et al. (2018) Carbon–TiO2 composites as high-performance supercapacitor electrodes: synergistic effect between carbon and metal oxide phases. Journal of Materials Chemistry A, 6, pp. 633–644. doi.org/10.1039/C7TA08023A.
- Ge, Y., et al. (2015) High cyclability of carbon-coated TiO2 nanoparticles as anode for sodium-ion batteries. Electrochimica Acta, 157, pp. 142–148. https://doi.org/10.1016/j.electacta.2015.01.086.
- Maldonado-Hódar, F. J., et al. (2000) Synthesis, pore texture and surface acid–base character of TiO2/carbon composite xerogels and aerogels and their carbonized derivatives. Applied Catalysis A: General, 203, pp. 151–159. doi.org/10.1016/S0926-860X(00)00480-4.
- Moreno-Castilla, C & Maldonado-Hódar, F J (2000) Synthesis and surface characteristics of silica– and alumina–carbon composite xerogels. Physical Chemistry Chemical Physics, 2, pp. 4818–4822. doi.org/10.1039/B004223O.
- Nunes Simonetti, E. A., et al. (2021) TiO2 as a gas sensor: The novel carbon structures and noble metals as new elements for enhancing sensitivity. A review. Ceramics International, 47, pp. 17844–17876. doi.org/10.1016/j.ceramint.2021.03.189.
- Raut, B. T., et al. (2012) CSA doped polyaniline/CdS organic–inorganic nanohybrid: Physical and gas sensing properties. Ceramics International, 38, pp. 5501–5506. doi.org/10.1016/j.ceramint.2012.03.064.
- Maldonado-Hódar, F. J., et al. (1999) Synthesis and textural characteristics of organic aerogels, transition-metal-containing organic aerogels and their carbonized derivatives. Carbon, 37, pp. 1199 –1205. doi.org/10.1016/S0008-6223(98)00314-5.
- Al-Muhtaseb, S A & Ritter, J A (2003) Preparation and Properties of Resorcinol–Formaldehyde Organic and Carbon Gels. Advanced Materials, 15, pp. 101–114. doi.org/10.1002/adma.200390020.
- Khatun, N., et al. (2017) Anatase to rutile phase transition promoted by vanadium substitution in TiO2: A structural, vibrational and optoelectronic study. Ceramics International, 43, pp. 14128–14134. doi.org/10.1016/j.ceramint.2017.07.153.
- Zhao, D., et al. (2020) C-doped TiO2 nanoparticles to detect alcohols with different carbon chains and their sensing mechanism analysis. Sensors and Actuators B: Chemical, 312, p. 127942. doi.org/10.1016/j.snb.2020.127942.
- Williams, D E (1999) Semiconducting oxides as gas-sensitive resistors. Sensors and Actuators B: Chemical, 57, pp. 1–16. doi.org/10.1016/S0925-4005(99)00133-1.
- Chen, H., et al. (2012) A comparative study on UV light activated porous TiO2 and ZnO film sensors for gas sensing at room temperature. Ceramics International, 38, pp. 503 –509. doi.org/10.1016/j.ceramint.2011.07.035.
- Anothainart, K., et al. (2003) Light enhanced NO2 gas sensing with tin oxide at room temperature: conductance and work function measurements. Sensors and Actuators B: Chemical, 93, pp. 580–584. doi.org/10.1016/S0925-4005(03)00220-X.
- Thangamani, G J & Pasha, S K K (2021) Titanium dioxide (TiO2) nanoparticles reinforced polyvinyl formal (PVF) nanocomposites as chemiresistive gas sensor for sulfur dioxide (SO2) monitoring. Chemosphere, 275, p. 129960. doi.org/10.1016/j.chemosphere.2021.129960.
- Wang, M., et al. (2020) Sol-gel derived TiO2–carbon composites with adsorption-enhanced photocatalytic activity and gas sensing performance. Ceramics International, 46, pp. 18608–18613. doi.org/10.1016/j.ceramint.2020.04.171.
- Liou, W J & Lin, H M (2007) Nanohybrid TiO2/carbon black sensor for NO2 gas. China Particuology, 5, pp. 225–229. https://doi.org/10.1016/j.cpart.2007.03.005.
- Kılınç, N., et al. (2014) Fabrication and gas sensing properties of C-doped and un-doped TiO2 nanotubes. Ceramics International, 40, pp. 109–115. doi.org/10.1016/j.ceramint.2013.05.110.