To deposit components that confer electrocatalytic and conductivity activity onto the paper substrate surface, conventional routes for producing paper-based electrochemical sensors employ post-functionalization methods.
Although these studies come with impressive performance, significant requirements for the widespread adoption of paper-based electrochemical sensors will be the scalable synthesis of reliable, cheap paper-based electrodes with repeatable properties, which remains a challenge.
A new study hopes to address the issues surrounding the scalable manufacturing and regulated functionalization of paper. Here, the team reports the use of this paper for selective and sensitive electrochemical detection depending on two outcomes. Firstly, they adapt traditional papermaking approaches to generate a functional composite paper made of cellulose fibers, graphene oxide (GO), and carbon fibers (CF).
As shown in Figure 1a, the effective fabrication of this composite needed interfacial engineering to regulate the communications among the hydrophobic CF, hydrophilic cellulose fibers, and nanosized GO fillers. Figure 1b shows that the study employed a relatively mild and efficient electrochemical treatment to decrease the GO in the composite to its reduced form (rGO) to make it work.
Figure 1c proposes that this composite paper can offer a way to understand the vision of cost-effective, paper-based electrodes for electrochemical detection.
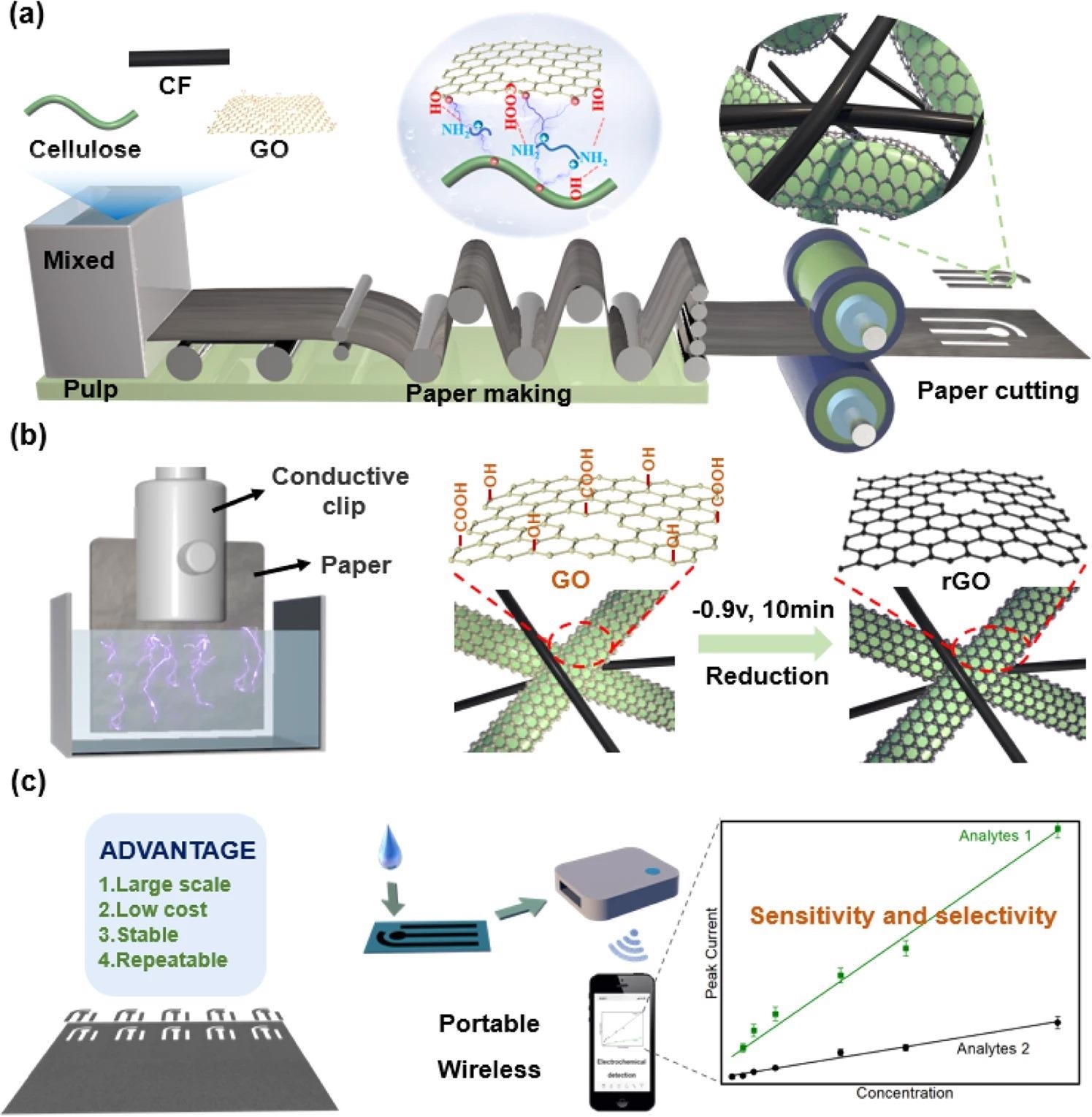
Figure 1. Large-scale preparation of high-performance paper-based electrochemical sensors. (a) Large-scale papermaking. By controlling interfacial interactions, composite paper was prepared composed of cellulose, carbon fibers (CF, confers conductivity), and graphene oxidize (GO, confers electrocatalytic activities). (b) Electrochemistry reduction of GO. Electrochemistry provides a simple scalable approach to GO reduction. (c) Vision of paper-based electrochemical sensor. The vision for high-performance and sustainable paper-based sensors for portable electrochemical detection. Image Credit: Wang, C., et al., 2022
Results and Discussion
A crucial requirement for employing papermaking technology to make uniform and stable composite paper with recurring properties is that the CF, cellulose, and GO must be evenly dispersed in water to prepare pulp (Figure 2). The GO and cellulose fibers are hydrophilic with huge negative charges, whereas the CF fibers are lightweight and hydrophobic with a small negative charge, as identified by the zeta-potential measurements in Figure 2b.
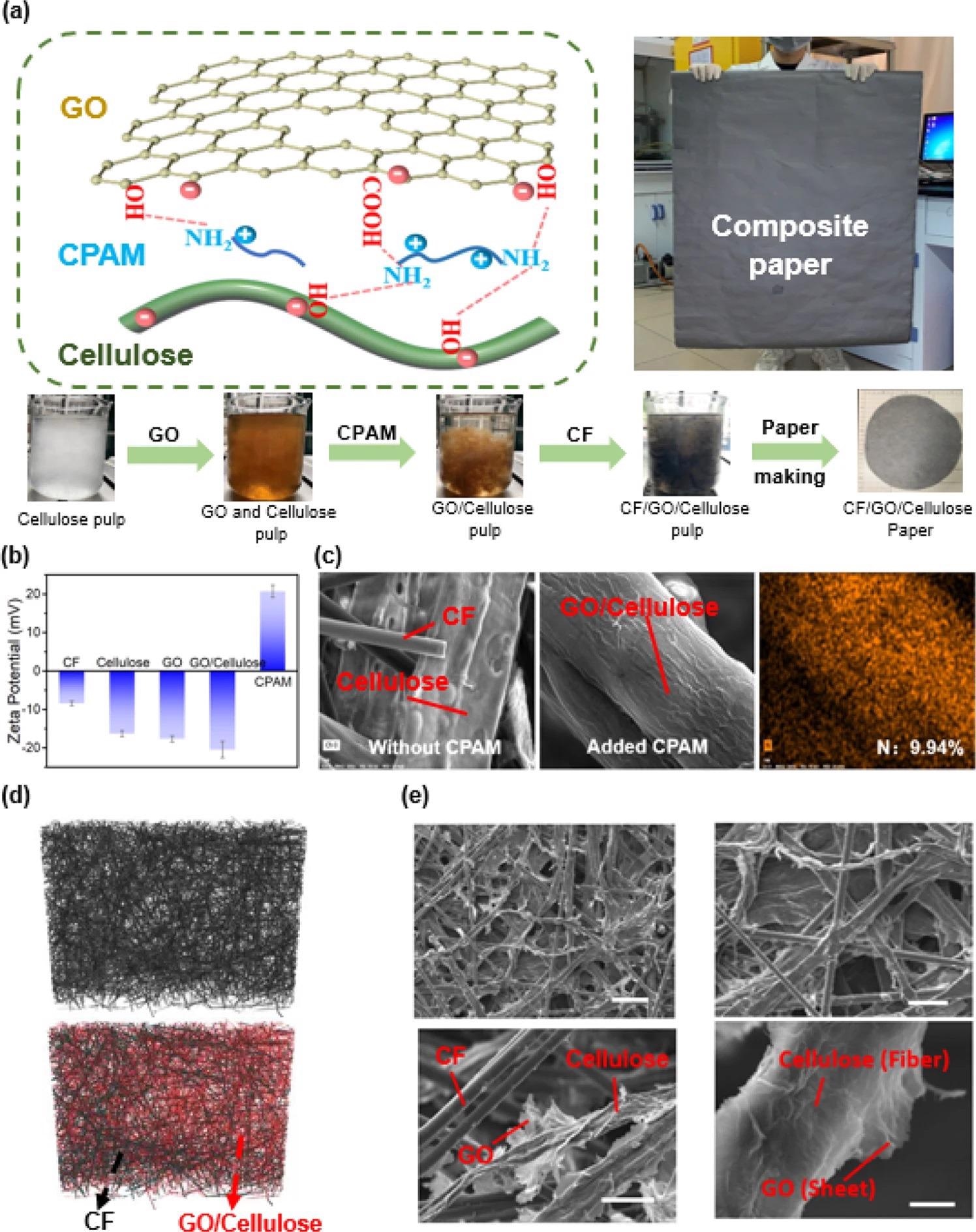
Figure 2. Preparation and characterization of CF/GO/cellulose paper. (a) Fabrication of CF/GO/cellulose paper. Cationic polyacrylamide (CPAM) was used to control the interfacial interactions to enable the assembly of GO sheets onto the surface of the cellulose fibers. (b) Electrostatic interaction. Zeta-potential measurements indicate the electrostatic charge of the components. (c) The distribution of CPAM was detected by EDS. EDS shows that CPAM (determined by nitrogen) is localization on the surface of cellulose. (d) 3D structure by micro-CT. Micro-CT shows a homogeneous distribution of the CF and cellulose fibers in the CF/GO/cellulose composite. (e) Surface morphology by SEM. Scanning electron microscopy images show the composite’s homogeneous distribution and also the assembly of the GO sheets on the cellulose surface. Scale bar is: top left 50 μm, bottom left 10 μm, top right 10 μm, and bottom right 1 μm. Image Credit: Wang, C., et al., 2022
The research team used three independent measurements to offer microstructural and molecular evidence for the efficient integration of components to create the composite paper. Firstly, energy dispersive X-ray spectroscopy (EDS) is used to offer proof for the site of the CPAM (as calculated by elemental nitrogen) in the composite paper. Secondly, micro-computed tomography (micro-CT) was used to analyze the carbon distribution and cellulose fibers inside the CF/GO/cellulose composite.
Thirdly, scanning electron microscopy (SEM) analysis investigated the composite paper’s microstructure. The results show that the study has efficiently prepared stable and uniform CF/GO/cellulose paper.
As illustrated in Figure 3a, the study employed a relatively mild electrochemical approach in which the composite paper was submerged in an electrolyte solution and a cathodic voltage was applied. Figure 3b depicts current–time (i–t) curves for the current drawn through many composite paper formulations.
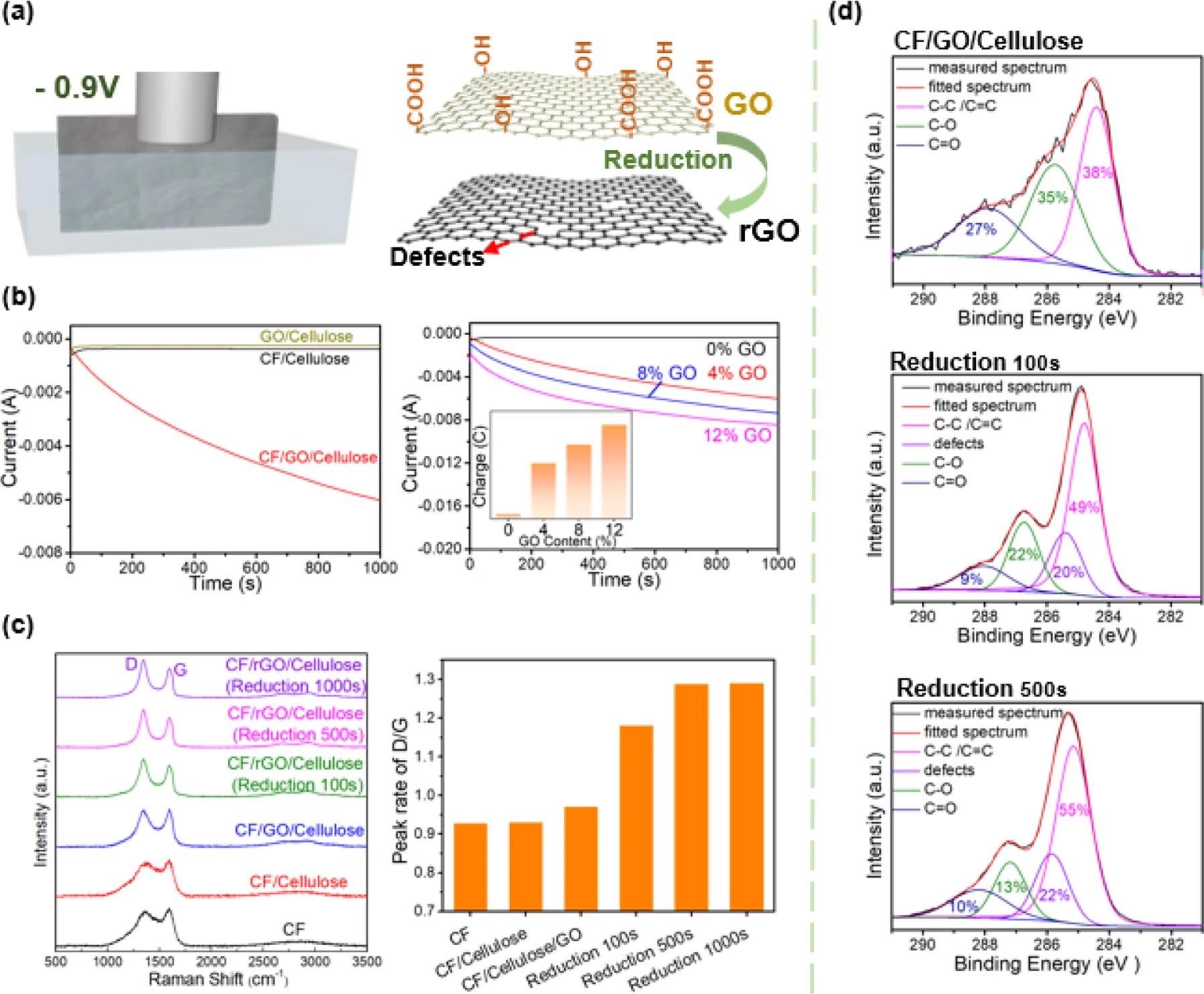
Figure 3. Electrochemical reduction of GO in the composite paper. (a) Electrochemical reduction of graphene oxide. Schematic of electrochemical conversion of GO to reduced GO (rGO). (b) Controllable electrochemical reduction of GO in paper. Electrochemical measurements of current vs time (i–t) show that CF is required for reduction (CF provides the conducting network) and the reducing charge transferred (Q=∫idtQ=∫idt) increases with GO content (GO is the species being reduced). (c) Raman evidence on GO electrochemical reduction. Raman spectroscopy provides molecular evidence for GO reduction and the generation of defects (indicated by D band) is believed to be important for electrocatalytic activity. (d) High-resolution XPS spectra of C 1 s. The high-resolution C 1 s peaks of XPS provide further molecular evidence for electrochemical reduction. Image Credit: Wang, C., et al., 2022
The summary bar chart and Raman spectra show the results for CF/GO/cellulose composites, which had been electrochemically reduced several times, along with the results from controls. These electrochemical and chemical measurements both show that the CF/GO/cellulose composite paper can be electrochemically reduced.
To demonstrate the macroscopic electrical properties of the composite paper, researchers used traditional cutting operations to make patterned “sensors” (Figure 4a). The images in Figure 4b offer a simple illustration that these composites own conducting properties. The schematic in Figure 4c depicts that these composites can also act as functioning electrodes in a three-electrode electrochemical cell.
![Important features of CF/GO/cellulose composite paper. (a) Cut paper. Paper cutting allows reproducible generation of different patterns. Scale bar is 2?cm. (b) Repairability. The composite paper is conducting (conductivity value is 1389?S?m-1). Electrochemical measurements [of K3Fe(CN)6] demonstrate that the CF/GO/cellulose composite is (c) flexibility (CV of potassium ferricyanide). Electrochemically active and flexible. (d) Stability. Structurally and functionally stable in water, and I repeatability. Repeatable in that different samples (i.e., different sensors) taken from different locations show the same functional performance.](https://www.azosensors.com/images/Article_Images/ImageForArticle_2466_16457808754465563.jpg)
Figure 4. Important features of CF/GO/cellulose composite paper. (a) Cut paper. Paper cutting allows reproducible generation of different patterns. Scale bar is 2 cm. (b) Repairability. The composite paper is conducting (conductivity value is 1389 S m−1). Electrochemical measurements [of K3Fe(CN)6] demonstrate that the CF/GO/cellulose composite is (c) flexibility (CV of potassium ferricyanide). Electrochemically active and flexible. (d) Stability. Structurally and functionally stable in water, and I repeatability. Repeatable in that different samples (i.e., different sensors) taken from different locations show the same functional performance. Image Credit: Wang, C., et al., 2022
The stability in water is one practically important feature of this composite paper. To demonstrate this water stability, researchers submerged the composite electrode in water. Sixty hours later, the composite paper did not show any evidence of leaching or swelling of the GO components.
Another practically vital characteristic that results from the fabrication method is repeatability. The findings show that composite paper provides significant practical characteristics for paper-based electrochemical sensing.
The sensors were assessed by the performance of CV measurements with K3Fe(CN)6, as illustrated in Figure 5a.
![Electrochemical detection by composite paper. (a) Electrocatalytic activities. Electrochemical reduction of CF/GO/cellulose confers electrocatalytic activity to the composite [as measured by CV measurements of Fe(CN)63-]. Differential pulse voltammetry (DPV) measurements with three phenols (hydroquinone, chlorophenol, and nitrophenol) illustrate that the electrocatalytic properties enhance (b) selectivity of electrochemical sensing. Detection selectivity (DPV-peak separation) and (c) sensitivity of electrochemical sensing. Detection sensitivity (peak amplification; error bars show the standard deviation from three measurements and are generally small relative to symbol size).](https://www.azosensors.com/images/Article_Images/ImageForArticle_2466_16457808851687292.jpg)
Figure 5. Electrochemical detection by composite paper. (a) Electrocatalytic activities. Electrochemical reduction of CF/GO/cellulose confers electrocatalytic activity to the composite [as measured by CV measurements of Fe(CN)63−]. Differential pulse voltammetry (DPV) measurements with three phenols (hydroquinone, chlorophenol, and nitrophenol) illustrate that the electrocatalytic properties enhance (b) selectivity of electrochemical sensing. Detection selectivity (DPV-peak separation) and (c) sensitivity of electrochemical sensing. Detection sensitivity (peak amplification; error bars show the standard deviation from three measurements and are generally small relative to symbol size). Image Credit: Wang, C., et al., 2022
As depicted in Figure 5b, hydroquinone (HQ) could be oxidized under the influence of mildly oxidative voltages, whereas p-chlorophenol (CP) and p-nitrophenol (NP) need more positive voltages for their oxidation. When solutions comprising a single phenol (10 mg L−1) were quantified, the DPV curves in Figure 5b depict that an oxidation peak was seen, and these oxidation peaks happened at varying voltages.
When a solution was made including all three phenols (10 mg L−1 for each phenol), Figure 5c presents three distinct oxidation peaks and the corresponding voltages which are consistent when compared to the individual phenols.
Researchers prepared the radar chart in Figure 6 to give a multivariate comparison of the composite paper with other demonstrative electrochemical sensors that have been taken into account for hydroquinone (HQ) analysis.
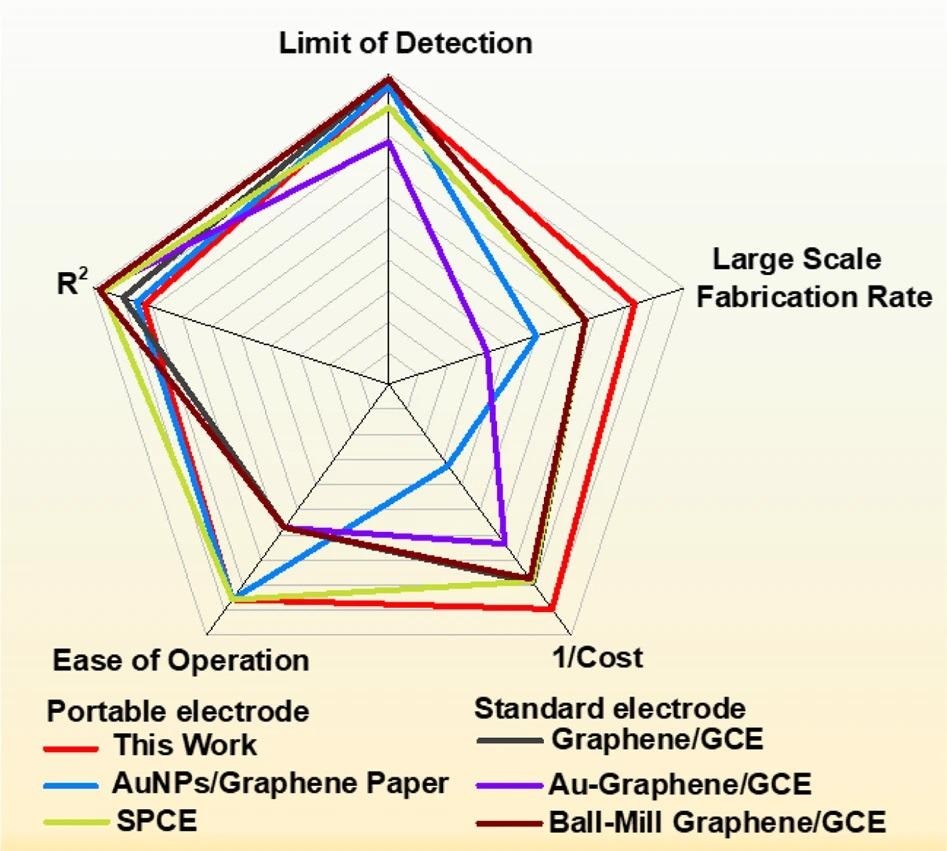
Figure 6. Comparison of CF/GO/Cellulose paper - based sensors with other sensors. Radar chart compares the manufacturability and performance of CF/GO/Cellulose paper-based electrochemical sensor with sensors from other studies. Image Credit: Wang, C., et al., 2022
Methodology
Graphene oxide (GO), cellulose pulp, carbon fiber (CF), cationic polyacrylamide (CPAM), Hydroquinone (HQ), p-chlorophenol (CP), p-nitrophenol (NP), and all other reagents were used as received with no further purification. Ultrapure water (>18 MΩ) was also used for experiments.
All test samples were made ready in the lab; scalable fabrication of composite paper is made by a pilot-scale paper machine in the same ratio with 50 kg of softwood pulp.
GO of the CF/GO/cellulose paper was reduced electrochemically with a three-electrode system, in which the reference electrode was Ag/AgCl, the counter electrode was a platinum plate, the reaction condition was −0.9 V, the electrolyte was 1 M KCl solution, and the reaction duration was varied, based on experiment 100–1000 s.
Cyclic voltammograms (CV) were executed along with electrochemical impedance spectroscopy. The phenols CP, HQ, and NP were dissolved in a 0.1 M phosphate buffer of pH 7 and detected with composite paper as the functioning electrode in a three-electrode system. Measurements were carried out with differential pulse voltammetry (DPV) with the probable range of 0–1.1 V.
Conclusion
Researchers report the preparation of a low-cost composite paper that provides electrocatalytic and conducting properties appropriate for electrochemical sensing. The team believed that there are three important characteristics of this work. First, regulating interfacial interactions is necessary for producing a composite from an equivalent amount of the cellulose and carbon fibers, and also for arranging the GO sheets onto the cellulose fibers.
Second, reduction of the GO discusses electrocatalytic actions to the composite. Hence, they performed the reduction with an electrochemical step that is scalable and mild (less than 1 V for several minutes).
Third, the composite paper has many useful features: it can be designed using seamless cutting operations, it is stable and flexible in water, and its electrocatalytic actions allow selective and sensitive chemical analysis. They envision that these features of the composite offer the occasion to appreciate the vision for low-cost, portable, and sustainable paper-based electrochemical detection.
Journal Reference:
Wang, C., Wu, C., Ling, H., Zhao, Z., Han, W., Shi, X., Payne, G. F., Wang, X. (2022). Toward scalable fabrication of electrochemical paper sensor without surface functionalization. npj Flexible Electronics, 6, p. 12. Available Online: https://www.nature.com/articles/s41528-022-00143-1.
References and Further Reading
- Liu, X., et al. (2019) A universal paper-based electrochemical sensor for zero-background assay of diverse biomarkers. ACS Applied Materials & Interfaces, 11(17), pp. 15381–15388. doi.org/10.1021/acsami.9b03860.
- Nonaka, L. H., et al. (2020) Crumpled graphene decorated with manganese ferrite nanoparticles for hydrogen peroxide sensing and electrochemical supercapacitors. ACS Applied Nano Materials, 3(5), pp. 4859–4869. doi.org/10.1021/acsanm.0c01012.
- Hafez, A. M., et al. (2019) Flexible lithium metal anode featuring ultrahigh current density stability with uniform deposition and dissolution.ES Energy & Environment, 5, pp. 85–93. doi.org/10.30919/esee8c311.
- Wang, Y., et al. (2019) Label-free microfluidic paper-based electrochemical aptasensor for ultrasensitive and simultaneous multiplexed detection of cancer biomarkers. Biosensors and Bioelectronics, 136, pp. 84–90. doi.org/10.1016/j.bios.2019.04.032.
- Sakar, M., et al. (2019) Graphene paper-based electrochemical sensors for biomolecules. Graphene-Based Electrochemical Sensors for Biomolecules, 1, pp. 297–320. doi.org/10.1016/B978-0-12-815394-9.00012-1.
- Liu, H., et al. (2020) Self-assembly of surface-acylated cellulose nanowhiskers and graphene oxide for multiresponsive Janus-like films with time-dependent dry-state structures. Small, 16, pp. 1–10.
- Li, T., et al. (2021) Developing fibrillated cellulose as a sustainable technological material. Nature, 590, pp. 47–56. doi.org/10.1038/s41586-020-03167-7.
- Wang, P., et al. (2017) Development of a paper-based, inexpensive, and disposable electrochemical sensing platform for nitrite detection. Electrochemistry Communications, 81, pp. 74–78. doi.org/10.1016/j.elecom.2017.06.006.
- Yakoh, A., et al. (2019) 3D capillary-driven paper-based sequential microfluidic device for electrochemical sensing applications. ACS Sensors 4(5), pp. 1211–1221. doi.org/10.1021/acssensors.8b01574.
- Cinti, S., et al. (2017) Sustainable monitoring of Zn(II) in biological fluids using office paper. Sensors and Actuators B: Chemical, 253, pp. 1199–1206. doi.org/10.1016/j.snb.2017.07.161.
- Yao, B., et al. (2013) Paper-based solid-state supercapacitors with pencil-drawing graphite/polyaniline networks hybrid electrodes. Nano Energy, 2, pp. 1071–1078 . doi.org/10.1016/j.nanoen.2013.09.002.
- Wang, F., et al. (2019) Paper-based closed Au-bipolar electrode electrochemiluminescence sensing platform for the detection of miRNA-155. Biosensors and Bioelectronics, 150, p. 111917 .doi.org/10.1016/j.bios.2019.111917.
- Jin, H., et al. (2015) Improved performance of asymmetric fiber-based micro-supercapacitors using carbon nanoparticles for flexible energy storage. Journal of Materials Chemistry A, 3, pp. 15633–15641. doi.org/10.1039/C5TA03576G.
- Han, Y., et al. (2019) Hierarchical bi-continuous Pt decorated nanoporous Au-Sn alloy on carbon fiber paper for ascorbic acid, dopamine and uric acid simultaneous sensing. Biosensors and Bioelectronics, 124–125, pp. 191–198. doi.org/10.1016/j.bios.2018.10.012.
- Ippolito, S. J., et al. (2005) Hydrogen sensing characteristics of WO3 thin film conductometric sensors activated by Pt and Au catalysts. Sensors and Actuators B: Chemical, 108(1-2), pp. 154–158. doi.org/10.1016/j.snb.2004.11.092.
- Yao, Y., et al. (2019) Spontaneous growth and regulation of noble metal nanoparticles on flexible biomimetic MXene paper for bioelectronics. Biosensors and Bioelectronics, 148, p. 111799. doi.org/10.1016/j.bios.2019.111799.
- Mai, V. P., et al. (2019) Porosity estimation using electric current measurements for paper-based microfluidics. Microfluidics and Nanofluidics, 23, p. 59. doi.org/10.1007/s10404-019-2226-x.
- Lee, D J & Kim, D Y (2019) Hydrophobic paper-based SERS sensor using gold nanoparticles arranged on graphene oxide flakes. Sensors, 19(24), p. 5471. doi.org/10.3390/s19245471.
- Fu, H., et al. (2019) A paper-based microfluidic platform with shape-memory-polymer-actuated fluid valves for automated multi-step immunoassays. Microsystems & Nanoengineering, 5, p. 50. doi.org/10.1038/s41378-019-0091-0.
- Wang, Q., et al. (2015) Cationic polyacrylamide enhancing cellulase treatment efficiency of hardwood kraft-based dissolving pulp. Bioresource Technology, 183, pp. 42–46. doi.org/10.1016/j.biortech.2015.02.011.
- Eduardo, A., et al. (2019) Novel electrochemical sensing platform based on a nanocomposite of PVA/PVP/RGO applied to IgG anti- Toxoplasma gondii antibodies quantitation. Talanta, 195, pp. 699–705. doi.org/10.1016/j.talanta.2018.11.070.
- Jalal, N. R., et al. (2020) In situ growth of metal–organic framework HKUST-1 on graphene oxide nanoribbons with high electrochemical sensing performance in imatinib determination. ACS Applied Materials & Interfaces, 12(4), pp. 4859–4869. doi.org/10.1021/acsami.9b18097.
- Li, X., et al. (2019) Ball‐Mill‐Exfoliated graphene: tunable electrochemistry and phenol sensing. Small, 15(48), p. 1805567. doi.org/10.1002/smll.201805567.
- Pw, A., et al. (2019) Defect-dependent electrochemistry of exfoliated graphene layers. Carbon, 154, pp. 125–131. doi.org/10.1016/j.carbon.2019.07.100.
- Shukla, R. P., et al. (2020) A reduced-graphene oxide-modified microelectrode for a repeatable detection of antipsychotic clozapine using microliters-volumes of whole blood. Talanta, 209, p. 120560. doi.org/10.1016/j.talanta.2019.120560.
- Randles, J(1948) A cathode ray polarograph. Part II.—The current-voltage curves. Transactions of the Faraday Society, 44, p. 327. doi.org/10.1039/TF9484400327.
- Zhou, M(2009) Electrochemical sensing and biosensing platform based on chemically reduced graphene oxide. Analytical Chemistry, 81, pp. 5603–5613. doi.org/10.1021/ac900136z.
- Shamkhalichenar, H & Choi, J W (2020) Review—non-enzymatic hydrogen peroxide electrochemical sensors based on reduced graphene oxide. Journal of The Electrochemical Society, 167(3), pp. 037531. doi.org/10.1149/1945-7111/ab644a.
- M. Ej Ri., et al. (2019) Reduced graphene oxide nanosheets modified with nickel disulfide and curcumin nanoparticles for non-enzymatic electrochemical sensing of methyl parathion and 4-nitrophenol. Microchimica Acta, 186, p. 704. doi.org/10.1007/s00604-019-3853-3.
- Tsksa, B &Kyhab, C (2020) Rational design and preparation of copper vanadate anchored on sulfur doped reduced graphene oxide nanocomposite for electrochemical sensing of antiandrogen drug nilutamide using flexible electrodes. Journal of Hazardous Materials, 410, p. 124659. doi.org/10.1016/j.jhazmat.2020.124659.
- Huo, Z., et al. (2011) Sensitive simultaneous determination of catechol and hydroquinone using a gold electrode modified with carbon nanofibers and gold nanoparticles. Microchimica Acta, 173, pp. 119–125. doi.org/10.1007/s00604-010-0530-y
- Du, H., et al. (2011) A voltammetric sensor based on graphene-modified electrode for simultaneous determination of catechol and hydroquinone. Journal of Electroanalytical Chemistry, 650(2), pp. 209–213. doi.org/10.1016/j.jelechem.2010.10.002
- Wang, Z., et al. (2007) Simultaneous determination of dihydroxybenzene isomers at single-wall carbon nanotube electrode. Sensors and Actuators B: Chemical, 127(2), pp. 420–425. doi.org/10.1016/j.snb.2007.04.037
- Ding, Y. P., et al. (2005) Direct simultaneous determination of dihydroxybenzene isomers at C-nanotube-modified electrodes by derivative voltammetry. Journal of Electroanalytical Chemistry, 575(2), pp. 275–280. doi.org/10.1016/j.jelechem.2004.09.020
- Ma, X. M., et al. (2013) Simultaneous determination of hydroquinone and catechol based on glassy carbon electrode modified with gold-graphene nanocomposite. Microchimica Acta, 180, pp. 461–468. doi.org/10.1007/s00604-013-0949-z
- Fan, L., et al. (2016) Disposable graphite paper based sensor for sensitive simultaneous determination of hydroquinone and catechol. Microchimica Acta, 213, pp. 504–511. doi.org/10.1016/j.electacta.2016.06.096.
- González-Costas, J. M., et al. (2020) Screen-printed electrodes-based technology: environmental application to real time monitoring of phenolic degradation by phytoremediation with horseradish roots. Science of the Total Environment, 744, p. 140782. doi.org/10.1016/j.scitotenv.2020.140782